Definition
A concentration gradient occurs when a solute is more concentrated in one area than another. A concentration gradient is alleviated through diffusion, though membranes can hinder diffusion and maintain a concentration gradient.
Overview
“Concentration” refers to how much of a solute is in a given amount of solvent. A corner of a water tank that has just had salt dumped into it would have a much higher concentration of salt than the opposite end of the tank, where no salt has diffused to. Therefore, a concentration gradient is said to exist in the tank.
Over time, solutes always move down their concentration gradient to “try” to produce an equal concentration throughout the whole solution. So, the concentration gradient above would eventually disappear as the ions of salt diffused throughout the entire tank.
The laws of thermodynamics state that due to the constant movements of atoms and molecules, substances will move from areas of higher concentration to lower concentration, in order to produce a randomly distributed solution. Water atoms like to completely surround each ion or polar molecule, which pulls them throughout a solution and separates them from one another.
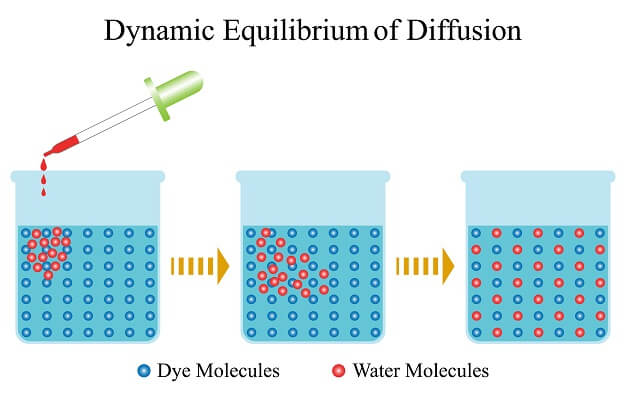
This can be easily demonstrated at home by adding a drop of food coloring to a glass of water. At first, the food coloring will only occupy the small spot in the water glass where it was added. But over time, the colored particles will spread, creating an equal distribution of colored particles throughout the bottom of the glass.
Function of Concentration Gradients
Concentration gradients are a natural consequence of the laws of physics. However, living things have found many ways to use their properties to accomplish important life functions. Concentration gradients are used by many cells to complete a wide variety of tasks. In fact, there is energy stored in a concentration gradient because the molecules want to reach equilibrium. So, this energy can be utilized to accomplish tasks.
It should also be noted that when a concentration gradient cannot be relieved through the diffusion of the solvent, osmosis may occur. Osmosis is the movement of water across a membrane and essentially does the same thing. Just like solutes are attracted to water, water is attracted to solutes. So, the concentration gradient can be alleviated by adding water to a highly concentrated membrane compartment (or cell).
Organisms that need to move a substance in or out of their cells may use the movement of one substance down its concentration gradient to transport another substance in tandem. This the basic method that protein antiporters and symporters use to bring crucial nutrients into cells. Organisms can also “harvest” the energy of the concentration gradient to power other reactions. See the examples below.
Examples of Concentration Gradients
ATP Synthase
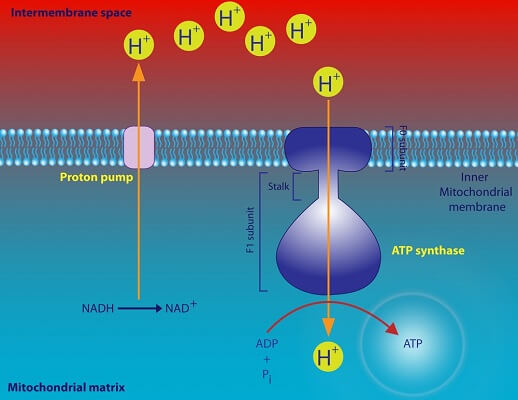
Some life forms use the tendency of solutes to move from an area of high concentration to low concentration in order to power life processes. ATP synthase – the protein that produces ATP – relies on a concentration gradient of hydrogen ions. As the ions pass through ATP synthase to cross the membrane and alleviate the gradient, ATP synthase transfers the energy into adding a phosphate group to ADP, thereby storing the energy in the newly formed bond.
Neurons and the Sodium/Potassium Pump
Neurons spend a huge amount of energy – about 20-25% of all the body’s calories, in humans – pumping potassium into their cells, and sodium out. The result is an extremely high concentration of potassium inside of nerve cells and a very high concentration of sodium outside. Since potassium
When cells communicate, they open ion gates that allow sodium and potassium to pass through. The sodium/potassium concentration differences are so strong that the ions “want” to instantly rush out of the cell. Because ions are electrically charged, this actually changes the electrical charge of the cell.
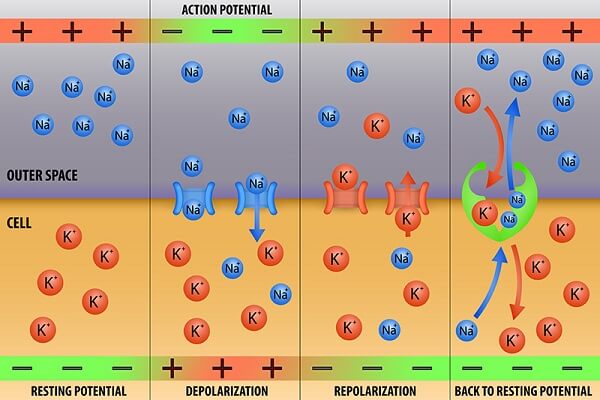
This “electrochemical” signal travels much faster than a merely chemical signal would, allowing us to perceive, think, and respond rapidly. Problems that interfere with the neurons’ sodium/potassium pump can cause death very quickly because the heart muscle itself relies on these electrochemical impulses to pump blood to keep us alive. This makes the sodium/potassium concentration gradient in neurons arguably the most important concentration gradient to human life!
Glucose/Sodium Symport Pump
The glucose-sodium symport pump also takes advantage of the sodium/potassium gradient.
One challenge faced by cells is moving glucose – which is large and difficult to move, compared to tiny sodium ions – and which often need to be moved against their concentration gradient. To solve this problem, some cells have “coupled” the movement of glucose with the movement of potassium, using proteins that will permit sodium to move down its concentration gradient – if it takes a glucose molecule with it.
This is just one more example of the ways in which cells use the basic laws of physics in innovative ways to accomplish the functions of life.
Lungs and Gills
The most common examples of concentration gradients involve solid particles dissolved in water. But gases can have concentration gradients, too.
Human lungs and fishes’ gills both use concentration gradients to keep us alive. Because oxygen follows the rules of concentration gradients just like any other substance, it tends to diffuse from areas of high concentration into areas of low concentration. That means that it diffuses from the air into our oxygen-depleted blood.
Lungs and gills make this process more efficient by rapidly running our most oxygen-depleted blood across the surfaces of our lungs and gills. This way, oxygen is constantly diffusing into the blood cells that need it most.
Quiz
1. Which of the following laws describes how concentration gradients work?
A. An object in motion tends to stay in motion, unless acted upon by an outside force.
B. Systems always progress toward a state of higher randomness.
C. Substances diffuse from areas of high concentration to areas of low concentration.
D. Both B and C.
2. Which of the following is NOT true of the sodium/potassium concentration gradient?
A. You can move a substance against its concentration gradient without expending energy, if you have the right transport protein.
B. Transport proteins that move substances against their concentration gradients need to be supplied with energy in order to function.
C. Because cells must break down molecules and expend energy, to move substances against their concentration gradient, this movement does not break the laws of thermodynamics.
D. None of the above.
3. Which of the following would we not be able to do if substances didn’t tend to move down their concentration gradients?
A. Think
B. Move
C. Breathe
D. All of the above
