Definition
HeLa cells, named after their original donor Henrietta Lacks, represent the most widely-used human cell line in the field of biological research. The cervical cells of a dying woman were kept alive (without consent) as ‘immortal’ cells in 1951 and fueled research into polio vaccination and isolation of the human immunodeficiency virus. They are still used today. Ethical issues behind the removal of tissues for research have only recently been approached; today, no medical professional may take or use tissue biopsies without the owner’s full, informed consent.
What Are HeLa Cells?
HeLa cells are cell lines, animal cell cultures removed from a living tissue during a biopsy and propagated ex vivo. HeLa cells are immortal cancer cells – they do not die but continue to divide when provided with nutrients. The primary HeLa cell culture – tissue from Henrietta Lacks’ uterus taken without her permission when she underwent treatment for cervical cancer in early 1951 – is alive even today. These are the daughter cells of the original cells taken from her womb.
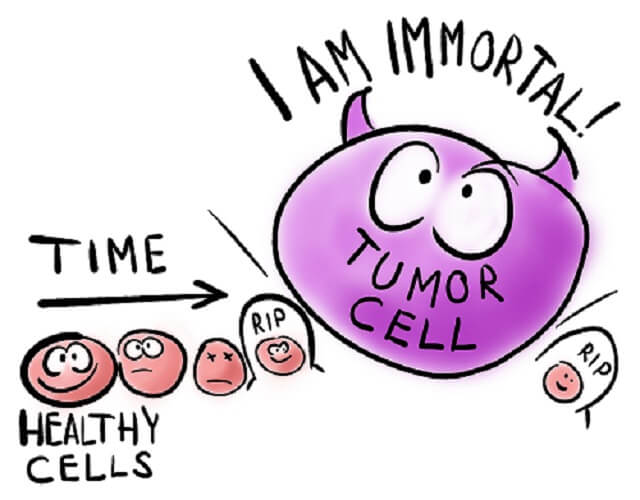
If healthy cells had been removed in Henrietta’s biopsy, they would have undergone controlled cell death at some point. Our cells are programmed as to how long they live. Cancer cells, however, are mutated cells that no longer respond to cell cycle regulation. They divide continuously. HeLa cells were the first immortal cell lines and have helped us to discover cures and treatments for many diseases.
Why Are HeLa Cells Immortal?
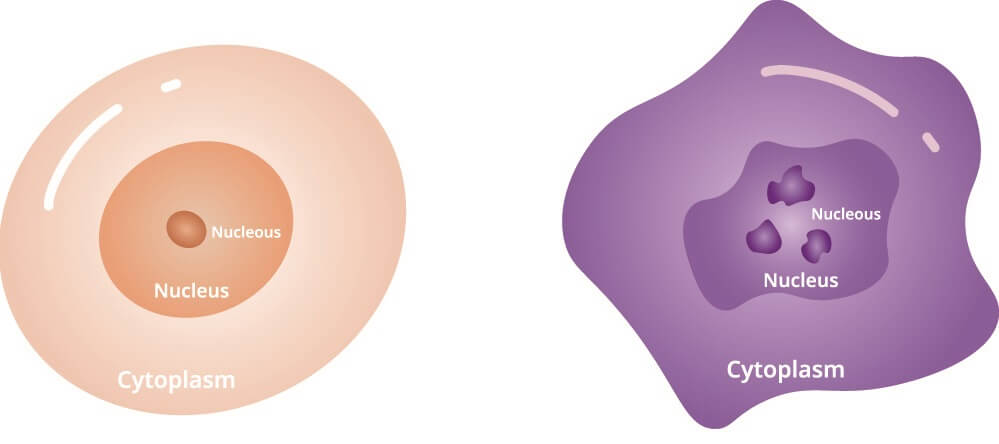
HeLa cells represent an immortalized cell line. As the definition of a cancer cell is a cell that, due to mutations in its DNA, does not die but continues to divide, only cancer cells provide scientists with immortalized cell lines. The HeLa cell definition, therefore, is a long line of cancer cells that grow outside of the body (in a laboratory) originating from tissue from Henrietta Lacks.
With genetic engineering making strides, it is now possible for geneticists to create their own continuous cell lines. By manipulating the genes that control cell death (apoptosis) and mitotic division, scientists can produce immortal cell lines from healthy cells. Researchers have already developed a line of near immortal stem cells (BEL-A cells) that form red blood cells when activated. These may eventually replace the need for blood donation – especially for rare blood types. Translating this technology to mass production is not yet possible. Furthermore, this is an artificial continuous but not immortal line. BEL-A cells eventually die off.
How a cancer cell achieves immortality is the subject of even more research. It seems that the telomeres of the chromosomes responsible for cell division and death elongate. In a healthy cell, telomeres shorten slightly with each cell division. Once they become a certain size, their other function as central DNA sequence protectors is lost. This allows the DNA to become so severely damaged the cell is either cleared away by the body’s immune cells or is forced to self-destruct.
Cancer cells and HeLa cells reverse this process. Rather than shortening over time, an immortal cell adds to its telomere at every division. This may be because the gene for telomerase production and activity remains switched on – the TERT and TERC genes are expressed in a mutated cancer cell. This very rarely occurs in normal cells and only for short periods.
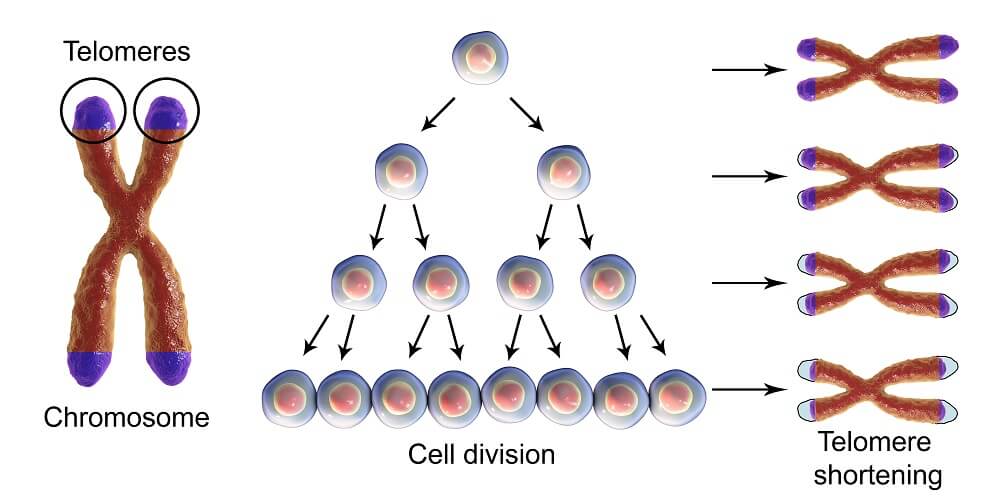
Telomeres
Telomeres are the equivalent of hard hats that protect our DNA. At the ends of each tightly-wound, double-helix DNA strand that forms individual chromosomes, you find the telomeres. Telomeres compose the ends of the arms (short and long) of the chromosome.
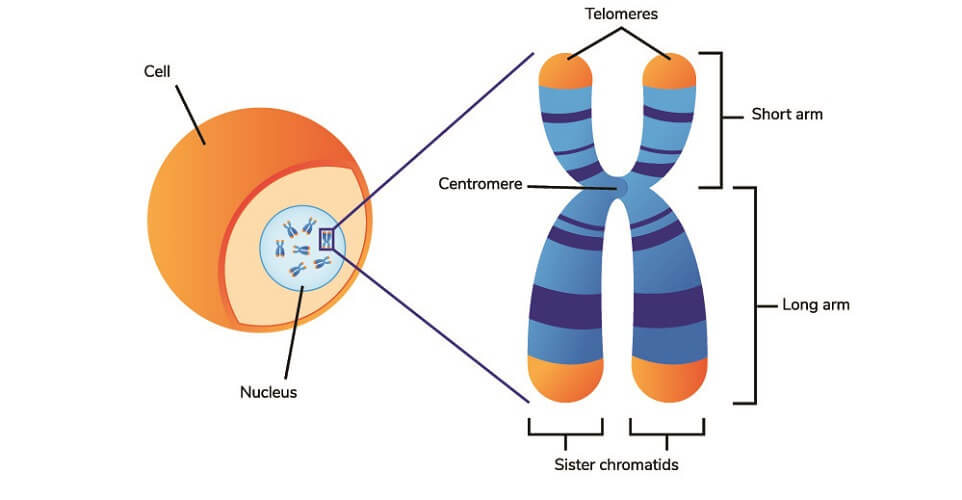
A telomere is a repeated non-coding DNA sequence. To date, we know that its function is to protect the DNA located deeper in the chromosome. Telomeres take the brunt of the attack from reactive oxygen species (ROS).
The longer the telomere, the longer the cell’s natural life. As we age, the telomeres in our cells shorten. Some tissues age more quickly than others, like the cells of the female reproductive tract. A long life can be hereditary – long telomeres can also be the result of our inherited genes.
Telomeres shorten when a cell divides. A cell that often divides will die more quickly than a slow-dividing cell. Mitosis requires DNA replication and every time this occurs, a telomere sequence is removed. Any damaged sequences within the exposed telomere will also be removed.
Telomerase
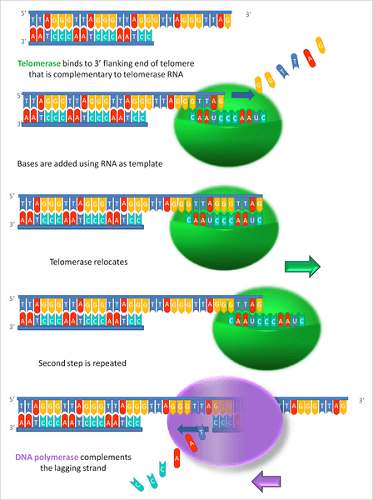
Telomerase is an enzyme that repairs the telomere of a chromosome. It slows the process of telomere shortening as damaged sequences are repaired rather than removed. However, the telomerase gene must be expressed before it can be produced.
Telomerase expression at the levels required to lengthen the telomeres only occurs in male germ cells (early sperm cell types), stem cells, blastocysts, and cancer cells. Other cells do not express telomerase at these high levels and are, therefore, susceptible to aging and eventually death.
Telomerase is comprised of a protein enzyme called telomerase reverse transcriptase (TERT) and an RNA fragment. The RNA component (TERC) provides the template that the penzyme follows to construct another telomere sequence.
The HeLa cell line does not have the longest telomeres of cancer cells. Those of the U2OS line extracted from a fifteen-year-old girl who died of osteosarcoma in the 1960s are much longer. Telomere lengths in normal cells are also relatively long; however, time reduces them.
Cancer Research
HeLa cell cancer research first asked scientists to make non-cancerous cells immortal. This has helped us to understand the mechanisms – such as the TERT gene mutation – that make cancer cells grow. Once the fundamentals of cancer gene variants are understood and we know more of the individual mechanisms, the next step is to make immortal cells die.
Up till now, killing immortal cells also means killing healthy cells. Only high levels of toxins can do the job, like chemotherapy. Radiation exposure also kills cancer cells with the risk of healthy cell DNA damage. The race is on to find individual products for cancer gene variants that act only on those mutations that affect cell lifespan and uncontrolled division. We still have a long way to go.
The HeLa Cell Line
The HeLa cell line has been used in tens of thousands of studies, some more important than others. Cells were even sent to space to look at the effects of zero gravity. HeLa cells used for cosmetic ingredient testing have helped to achieve huge profits for cosmetic companies. More importantly, they are still used to look at the results of different genetic variants of our DNA, treat viruses, develop and test new medicines, help us to understand how COVID-19 infects human cells, and bring us closer toward a cure for cancer.
HeLa cells versus normal cells features the following anomalies:
- HeLa cells contain between 76 to 80 chromosomes rather than 46. This is the result of the human papillomavirus that eventually killed Henrietta Lacks. Most viruses insert their own DNA into infected cells. This DNA produces a protein that stops DNA repair mechanisms.
- HeLa cells – and any ‘natural’ or artificial immortal cell – divide continuously and do not die if nourished.
- While most cancer cells do not grow at a quicker rate than normal cells, this is not true of HeLa cells. Henrietta had a particularly aggressive cancer type and also suffered from syphilis that compromised her immune system. This has made her cells extremely helpful in the laboratory as they proliferate so quickly.
Henrietta Lacks – HeLa
Henrietta Lacks was a poor African-American woman living at a time of severe racial inequality. When she could no longer ignore her abdominal pain, she had to travel to find a doctor willing to diagnose and treat her. This doctor was Dr. Howard Jones. To stage her cancer he carried out a biopsy.
However, without her knowledge, some of this tissue was given to a cancer researcher called Dr. George Gey. At the time, growing cells out of the body was a hit-and-miss affair. Gey found that Henrietta’s cancer cells proliferated outside of the womb at a rate no one had seen before. Gey continued to grow her cells in the laboratory even after her death a few, short months later. She was thirty-one years old.

Not only did Henrietta’s children, grandchildren, and great-grandchildren know nothing about this, they did not realize that Henrietta’s genome had been sequenced and made public. Only in 2013 did the National Institute of Health agree to release Henrietta’s genome sequence on an application basis only. Those who receive permission are obliged to include an acknowledgment of her family’s consent. At the same time, it was also ruled that naturally occurring genes can not be registered as patents. Although companies using HeLa cells in their research have profited from the results, no member of Henrietta Lacks’ family has received financial compensation.
HeLa cells represent two great strides in the field of scientific research. The building of cell lines that help to fight diseases like that which ended Henrietta’s life, and the change in the law that gives any US citizen (or European citizen) full rights over their body, from tissue to cell.

