Definition
Hydrochloric acid is a corrosive acid produced through the dissolving of hydrogen chloride (HCl) in water and is, therefore, an aqueous hydrogen halide solution. Hydrochloric acid is used in various industries as a cleaning, pickling or pH-adjusting solution and is also found in a diluted form in gastric juice. Hydrogen chloride is sometimes referred to as muriatic acid, hydronium chloride, chlorane, spirits of salt or acidum salis.
Hydrochloric Acid Uses
Hydrochloric acid uses range from the physiological to the industrial. In industry, the strong corrosive properties of HCl and its being the simplest chlorine-based acid make it a cheap and effective, if dangerous and destructive, compound. The aqueous solution must be pressurized or cooled or it will turn into a noxious gas should water components evaporate and number less than 60% of the total solution. While a complete list of hydrochloric acid use would fill many pages, the most common uses are discussed in more detail below.
In Industry
Industrial hydrochloric acid use spans a wide variety of sectors. It is an important ingredient in finished manufactured goods such as polyvinyl carbonate (PVC), bisphenol A (BPA), and ethylene dichloride (EDC). BPA is used in the production of polycarbonate plastics and epoxy resins but has been the source of a health scare due to carcinogenic effects. For this reason, many plastics are now manufactured and advertised as BPA-free products. All of these organic compounds contribute to the plastics industry and as laboratory solvents but can be highly carcinogenic.
Preparing metal for further manufacture involves one of two similar processes, pickling, and passivation. While passivation proffers a lighter effect, using lightly corrosive acids to remove the impurities left behind during metal manufacture, protect metal from pollutants, smooth the surface, and increase durability, pickling results in a stronger effect through the use of a strong acid. This acid is usually hydrogen chloride. Metals treated in hydrogen chloride baths are referred to as HCl-pickled metals.
Hydrochloric acid is often used in a single stage of leather processing at leather tanneries. Tanning hides involves the introduction of a chrome salt to change the collagen network within and so avoid future staining. During this process, chrome salts are added to a bath at a pH of between 2.5 and 3, together with sodium chloride. This is the pickling stage. The chrome is then fixed to the hide which increases the pH and is so known as the basification stage. In order to reach the low pH of the pickling stage, hydrogen chloride is necessary.
Another hydrogen chloride use in industry is the production of inorganic compounds such as poly aluminum chloride (PAC), iron (III) chloride (iron trichloride) and aluminum chlorohydrate. The use of hydrogen chloride is necessary at the start of the production processes of these compounds, for example the addition of 20% HCl and sulfuric acid to bauxite for PAC production. Aluminum salts are used in the cosmetic industry (such as in antiperspirant deodorants) or in the chemical coagulation and flocculation phases of potable and wastewater treatment where added aluminum (or iron) cations neutralize the charge of pollutant colloidal particles and allow them to join to form flocs (flocculation) they can be siphoned off.
HCl is also used to ‘purify’ the salt used in food production and on our tables. In a saturated NaCl solution, a constant equilibrium between separate positive sodium and negative chloride ions and unionized NaCl is present. By passing HCl gas through this solution it dissociates to form H+ and Cl- ions. As the chloride ion is common to both sodium chloride and hydrogen chloride, its concentration increases and creates a shift according to Le Chatelier’s principle. This means that available sodium ions are more likely to bind with the freely available chloride with increased NaCl deposits as a result. This process is called salt purification.Alternatively, you can add hydrochloric acid to sodium hydroxide (NaOH) or sodium carbonate (Na2Co3) and both reactions will form sodium chloride salt.
Naturally, a strong acid such as hydrogen chloride is also used to regulate the acidity of a wide range of solutions used in pharmaceutical products, in foods additives including fructose, citric acid, and hydrolyzed vegetable protein, in alkaline waste substances, and in our drinking water.
Hydrochloric acid is also used to increase oil production in oil wells. When injected into the base rock it produces larger pores that are able to transport more oil into the well. These are just some of the more common uses of hydrochloric acid in industry.
In the Home
Hydrochloric acid uses in the home are limited to lower concentrations that are less corrosive but still exhibit admirable cleaning and pH-adjusting characteristics. Those with swimming pools may want to try ten-part water to one-part HCl solution to remove tile grout stains.
The same solution will remove stains from metals and is therefore used in products sold to clean iron, copper, brass, and other metals. The effects are similar to steel pickling, oxidizing surface layers to remove stains and impurities. Most acids, including hydrogen chloride, will also remove lime scale deposits but attention must be paid to the concentration used. Most of the powerful cleaning products we use in the home contain HCl. If you spill hydrochloric acid on a delicate surface add a paste of bicarbonate of soda and water to neutralize it; have it pre-prepared and close at hand to avoid maximum damage.
Acid base reactions are often used to create a fizzing reaction that is said to increase the power of an acidic cleaning agent; however, you are only speeding up a reaction by adding an alkali where ideally the added acid should be working to neutralize the alkalines on the dirty surface. Hydrochloric acid will react with most carbonates and metals, such as with calcium carbonate to form calcium chloride, carbon dioxide and water or with magnesium which produces magnesium chloride, carbon dioxide and water.
In the Human Body
The stomach is the site of the early stages of digestion but has a similarly important role where potential pathogenic microorganisms that may have been ingested are eliminated due to a highly acidic environment of between 1.5-3.5 pH. This level of acidity is the result of gastric hydrogen chloride production.
In the human body, HCl is produced by the parietal cells of the stomach lining. The parietal cell cytoplasm combines with water and carbon dioxide to produce carbonic acid. The enzyme carbonic anhydrase converts one carbonic acid ion into a single hydrogen ion (H+) and a single bicarbonate ion (HCO3–). The hydrogen ion is transported into the stomach via the H+– K+ ATPase channel, exchanging positive extracellular potassium ions with positive intracellular hydrogen ions. At the same time, the bicarbonate ions are transported out of the cell into the blood via an anion exchanger that exchanges bicarbonate ions for negative chloride ions. Parietal cells also have chloride channels in their membranes. Negative chloride ions are transported into the stomach when intracellular concentrations increase.
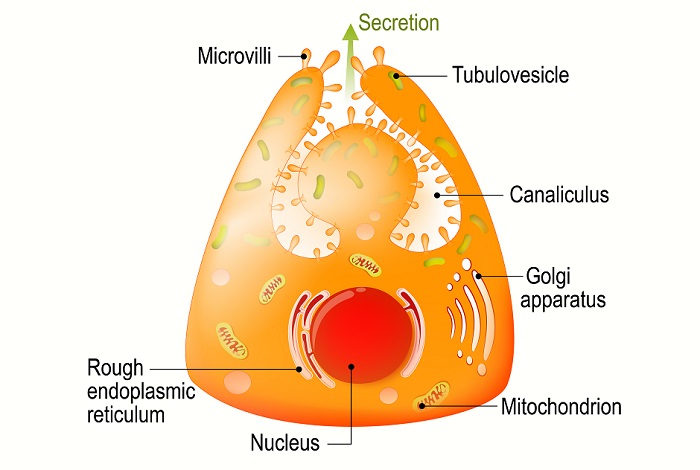
This process results in the presence of hydrogen and chloride ions in the stomach. As negative and opposite ions, they attract and form hydrochloric acid. Hydrochloric acid is an important component of gastric juice necessary to eliminate a broad range of potentially pathogenic bacteria before the stomach contents work their way into the highly absorbent environment within the intestines.
The human body can regulate hydrochloric acid production due to involuntary neuronal stimulation, firstly during vagus nerve stimulation when food is seen or chewed. As food reaches the stomach and the stomach stretches, resulting nerve firings also stimulate the vagus nerve to produce more acetylcholine which increases secretions such as saliva and gastric juice and gives more power to intestinal peristalsis. The third and main method is the secretion of gastrin from the stomach lining’s G-cells, similarly activated by the vagus nerve but also by peptides produced by the stomach such as gastrin-related peptide. Gastrin is a hormone that travels via the blood to the parietal cells where they bind to cholecystokinin (CKK) hormones by way of CKKB receptors and is part of the gut-brain link that controls satiety and appetite.
With the increased production of gastrin and acetylcholine, yet another reaction takes place in the form of histamine release from enterochromaffin-like cells (ECL cells) that lay close to the parietal cells in the stomach lining. This histamine binds to receptors on the parietal cells, encouraging them to produce more gastric acid.
The opposite effect – a decrease in hydrochloric acid production – occurs through starvation periods where no food is present in the stomach and acidity increases. High acidity halts hydrogen and chloride ion excretion in the parietal cell. It also stimulates D-cells to produce somatostatin that lowers the production of the hormone gastrin. Food passing into the duodenum also triggers a reflex known as the enterogastric reflex, a nerve pathway of the enteric nervous system that decreases vagal nerve stimulation. Food in the intestine also lowers the availability (and therefore the action) of cholecystokinin as well as secretin that reduces the production of acidic components of gastric juice and elevates production of alkaline components. It can, therefore, be safely said that the secretion of hydrochloric acid in the digestive system is regulated by hormonal and neural pathways that include gastrin, histamine, somatostatin, a range of polypeptides, and the vagus nerve.
In Warfare
Hydrochloric acid uses in warfare is most commonly associated with the production and effects of mustard gas or sulfur mustard used in trench warfare during the First World War, yet it has been recently used in the civil war in Syria, even though the Chemical Weapons Convention banned its use in 1993. Usually not fatal unless exposure occurs in high concentrations at regular intervals, this blistering agent attacks the skin and mucous membranes of the respiratory tract and digestive tract causing burns, swelling, irritation, and pus-filled blisters. Mustard gas also causes mutations in the DNA and is a known carcinogen. There is no antidote.
Various mustard gas production methods are used but only one of them – the Meyer-Clarke method – uses concentrated hydrochloric acid.
Another gas used in warfare is phosgene which has the formula COCl2. Unlike mustard gas that has a slightly greenish-yellow tinge, phosgene is colorless and smells like freshly cut hay. It is the result of carbon monoxide, activated charcoal, and chlorine gas. Hydrochloric acid is not used in phosgene production but is created in the presence of water. Water is found in large quantities in the mucous membranes. This means that when phosgene is inhaled or ingested it is converted into carbonic and hydrochloric acid. While mustard gas led to a number of fatalities, it is said that at least 80,000 WWI fatalities were due to the harsher and more immediate effects of phosgene. This is approximately 85% of the total chemical warfare-related deaths of this period.
Hydrochloric Acid Facts
Hydrogen chloride is a compound composed of a one-to-one ratio of hydrogen and chlorine. Without the presence of water molecules, hydrogen chloride is a colorless but toxic gas. Upon adding water, hydrogen releases many of its hydrogen molecules to produce a highly acidic solution. Just over 97% of the molecular mass of HCl is due to the single chloride ion. This chloride ion has an atomic mass of 35.543 and the hydrogen ion an atomic mass of 1.00794. As there is only one atom of each, the molar mass of HCl is calculated by adding these two figures together – 36.46094 g/mol. As already mentioned, the formula of hydrogen chloride is HCl.
In the case of molecular weight, results depend upon the number of moles of HCl. For example, in a solution where there are plenty of chloride dissolved gas atoms available (Cl2) for the number of molecular hydrogen atoms (H2), we can safely say that 4 moles of HCl will produce 4.00 mol HCl.
Using the equation Mass HCl = Moles HCl x Molar mass HCl we can determine that 4.00 mol x 36.46 g mol-1 is 146 g.
Hydrochloric acid density, pH, melting point and boiling point depends upon the concentration. For example, a 10% HCl solution has a density of 1048 kg/L, a pH of -0.5, a melting point of -18°C and a boiling point of 103°C. A 30% HCl solution has a density of 1.149 kg/L, a pH of -1, and a melting point and boiling point of -52°C and 90°C respectively.
Handling Hydrochloric Acid – MSDS Advice
Hydrochloric acid is a hazardous liquid and has its own material safety data sheet (MSDS). This is information that lists the occupational safety and health factors related to its use and must be available and easy to locate where potentially hazardous materials are found.
Hydrochloric acid is corrosive; concentrated forms also release toxic acidic mist. If either acid or mist comes into contact with the skin, eyes, or internal organs, any damage may be irreversible or perhaps fatal. Although HCl is not classified as a carcinogen, its industrial use requires personal protective equipment such as a vapor respirator, rubber gloves and boots, and a face shield. In addition, any premises where hydrochloric acid is used should have access to an eye-flush system. Even when cleaning at home with diluted products, splashes onto the eyes or skin can cause burns.
MSDS advice for hydrochloric acid contact with skin consists of rinsing the area for at least 15 minutes and removing any items of clothing that have come into contact with the solution. Where burns are visible, washing with antibacterial soap or using antibacterial cream is recommended, as is a visit to a medical center. Contact with the eyes requires a flushing system that rinses the affected eye for at least 15 minutes and requires medical attention.
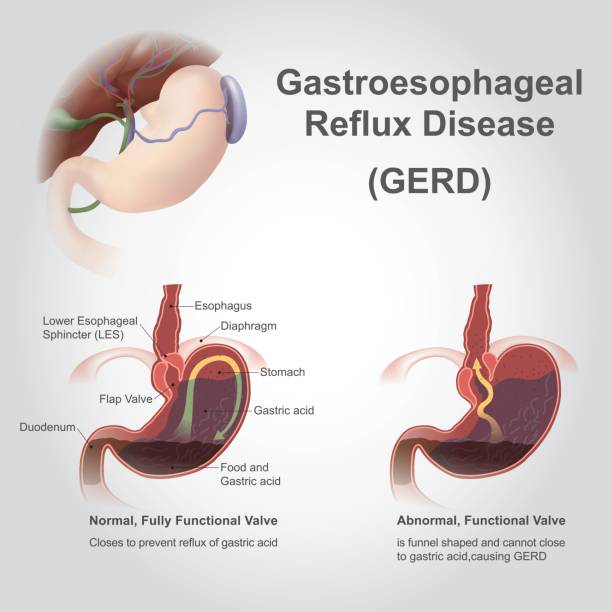
Ingesting hydrochloric acid of any concentration can cause burns to the inside of the mouth, throat, and esophagus. Even though gastric juice is highly acidic it remains within the reservoir of the stomach. Indigestion caused by gastric acid rising into the lower esophagus can cause Barrett’s esophagitis and increases the risk of esophageal cancer. A common disorder of the gastrointestinal tract is gastroesophageal reflux disease or GERD. The image below shows the signs and symptoms of this often painful pathology. If HCl is swallowed it is important not to vomit but instead to seek immediate medical attention. Inhalation of HCl mist also requires a visit to the emergency department.
MSDS storage information insists that hydrochloric acid must be stored in a closed and appropriate container in a cool, dry, and well-ventilated area. It should also be kept away from organic materials, oxidizing agents, metals, and alkalis as it can react with these and increase the concentrations of flammable hydrogen gas.
Quiz
1. Which of the following organic compounds is being phased out of the plastics industry?
A. Phosgene
B. BPA
C. Sulfuric acid
D. HCl
2. What should you first do if you ingest hydrogen chloride?
A. Drink lots of water
B. Vomit
C. Drink a bicarbonate solution
D. Seek medical attention
3. Where do coagulation and flocculation occur in terms of HCl?
A. Pickling metals
B. Water treatment
C. Treatment of burns
D. Salt purification
4. Which cells produce gastrin?
A. Parietal cells
B. T cells
C. G cells
D. ECL cells
5. Which cranial nerve is most involved in the regulation of gastric juice production?
A. Vagus nerve
B. Abducens nerve
C. Hypoglossal nerve
D. Oculomotor nerve
