Definition
Acetic acid is a mildly corrosive monocarboxylic acid. Otherwise known as ethanoic acid, methanecarboxylic acid, hydrogen acetate or ethylic acid, this organic compound is used in chemical manufacturing, as a food additive, and in petroleum production. The molecular formula of acetic acid is C2H4O2 or CH3COOH, where –COOH defines the presence of the single carboxyl group.

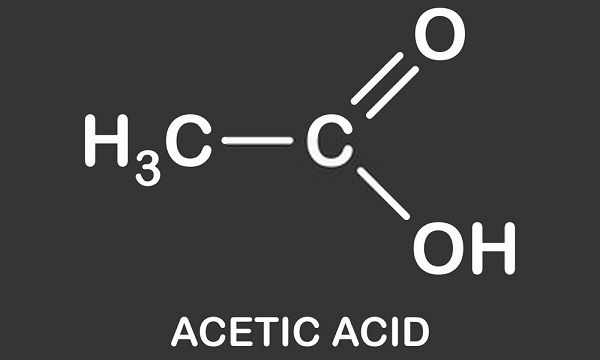
Acetic Acid Structure
Acetic acid structure is that of a simple carboxylic acid and consists of a methyl group attached to a carboxyl group as seen in the image below. Acetic acid or ethanoic acid is a protic solvent; it is able to donate protons in the form of hydrons (positively charged hydrogen atoms). This characteristic means it is a member of the Brønsted acid group where protons are donated to acceptor molecules known as Brønsted bases. The donated hydrogen is dissociated from the carboxyl group. Vinegar is a solution of acetic acid and water where approximately 0.4% of acetic acid molecules give up their H+ atoms leading to an acidic solution of approximately 2.4 pH. In comparison with the world’s strongest acid – carborane acid (H(CHB11Cl11)) – with a pH value of -18, acetic acid is mildly acidic in comparison.
It should be made clear that it is not the presence of a single hydrogen atom that changes the pH of a solution. Neutral solutions (neither acid nor alkaline) contain a balanced number of hydronium ions (H30+) and hydroxyl ions (OH–). Two molecules of water (H20) are formed when a hydronium and hydroxyl ion bind and the positive and negative charges are canceled out. When acetic acid is added to water, it splits into a negatively charged acetate ion (CH3COO–) and H+. It is, therefore, possible to understand the alternative name of acetic acid – hydrogen acetate. A small percentage of positively charged hydrogen ions bind to the water molecules and turn them into H30+. This means there are more hydronium ions and, therefore, create a positively charged (or acidic) solution. The pH of a solution is, therefore, dependent upon the balance of hydronium and hydroxyl and not the number of hydrogen ions, although these will affect this balance. A pH value is also only given to a solution. A solution always contains water; even modern superacids such as carborane are dissolved in concentrated aqueous solutions of other acids. Even glacial acetic acid has a small quantity of water.
The following image shows the dissociation of acetic acid to acetate in water. To the left are a single acetic acid molecule and a single water molecule. Acetic acid passes on a hydrogen ion to the water molecule to produce a hydronium ion. We say that the water molecule is protonated or has had a proton (hydron) donated to it.
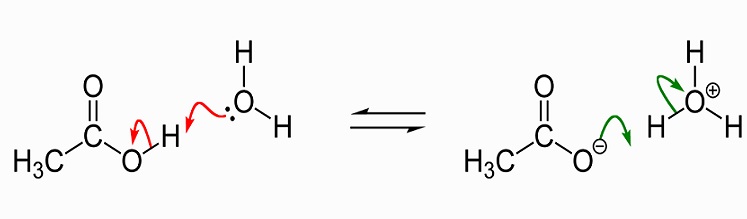
As a solvent, liquid acetic acid dissolves polar (hydrophilic) compounds such as salts and sugars and non-polar compounds which include fats and oils. This means it has many uses in industrial chemical production but has also gained a reputation as a weight-loss supplement as it affects fat and sugar metabolism. More information pertaining to acetic acid uses will be discussed later on in this article. In crystalline form, two acetic acid molecules join together with hydrogen bonds to form a dimer. When water is added, these bonds are broken and the crystalline form dissolves.

Acetic Acid Formula
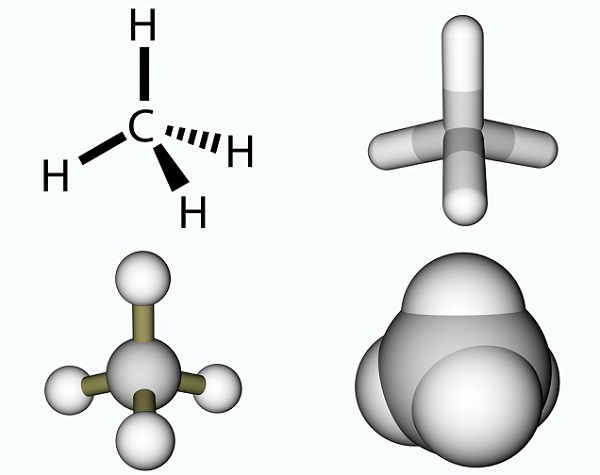
The acetic acid formula is a simple one and the result of a methyl group and a carboxyl group. Methyl groups are one of the most common organic compounds on the planet but are rarely found as single entities. They are composed of three hydrogen atoms and one carbon atom (CH3). As carbon has four electrons, the free electron usually bonds with other molecules by way of a covalent bond. The simplest carbon molecule is methane (CH4), well known for its contribution to global warming. With a free electron, methane reacts with ozone (O3) to produce carbon dioxide and water in the following reaction: (3)CH4 + (4)O3 = (3)CO2 + (6)H2O. The picture shows a methane molecule composed of a methyl group and an extra hydrogen atom.
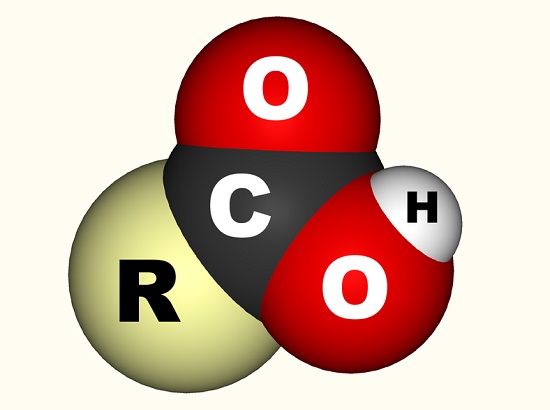
In the case of acetic acid, the free electron binds with a carboxyl group (CO2H, -COOH or -C(=O)OH) which is a single carbon atom bonded to a hydroxyl group (-OH) and double-bonded to an oxygen atom. The below image shows a carboxyl group where R represents the rest of the molecule to which the carboxyl group is attached; the letter R is sometimes replaced by a wiggly line. In the case of acetic acid, the R represents the methyl group. Some prefer to describe the carboxyl group as a combination of a carbonyl group (C=0, where = indicates the double bond) and a hydroxyl group (O-H). Carboxylic acids are found in amino acids and essential to every living organism.
There is a general molecular formula for all carboxylic acids, namely CnH2n+1COOH. This means that every carboxylic acid features twice as many hydrogen atoms as carbon atoms once the carboxyl group is removed; a formula that fits in perfectly with that of acetic acid – C2H4O2. When you remove the carboxyl group from this acetic acid formula, you are left with one carbon and two hydrogen atoms.
Acetic Acid Facts
Acetic acid facts for those that want to learn a little more!
The molar mass of acetic acid is 60.052 grams per mole (g/mol). Molar mass is the total mass of an element or compound (atomic mass) measured in atomic mass units or ‘amu’, divided by its amount in moles (mol). A single mole is based upon the Avogadro number 6.02214076×1023 as this number means that comparison between moles and Daltons, another scientific unit of atomic mass, is simpler.
Glacial acetic acid is a solution of acetic acid in a very small amount of water – less that 1%. The word glacial refers to its crystal-like solid form at room temperature. Another name for glacial acetic acid is anhydrous acetic acid. This form is a weak acid but a corrosive poison, causing blistering and burns. As there is very little water with which to dissociate, glacial acetic acid will pass on its protons to the water in the skin or mucous membranes.
Finding the right buffer agent for an acid such as acetic acid requires knowledge of the pH, Ka or pKa of the acid. The pH, Ka and pKa are all related to one another. Acetic acid has a Ka of 1.8 x 10-5 or an easier to calculate pKa value of 4.756. The pH measures the number of hydrogen ions (H+) in any solution that contains water and ranges from 0 (acidic) to 14 (base). The lower the pH, the higher the concentration of hydrogen ions. The Ka and pKa relate to acids and relate to the acid dissociation constant which shows how likely the acid is to give up its protons. A high Ka tells us that an acid is strong and will react to any chemical added to it. The pKa is the opposite – the smaller the number, the stronger the acid. This is because the pKa is a negative logarithm of the Ka.
However, concentrated acetic acid can have a lower pH than a strong acid. Thanks to the pKa which is a constant value, we can make calculations without having to think about concentrations. The pKa of acetic acid is 4.756 and this tells us how likely it is to give up its protons in a solution. Bases are measured according to how likely they are to remove protons from a solution.
The boiling point of acetic acid is between 244 and 246°F (118 and 119°C) and its melting point lies between 61 and 62°F (16 and 17°C) or just under room temperature. Acetic acid’s density is 1.049 g cm−3 in a liquid state, and 1.27 g cm−3 in a solid state.
The most commonly recognized form of acetic acid is vinegar which contains 5-20% acetic acid. How great the dilution is (and therefore the acid’s strength) is referred to as its grain strength. You can easily calculate this by multiplying the concentration by 10. Vinegar containing 5% acetic acid will have a grain strength of 50.
Uses of Acetic Acid
Acetic acid uses are many and varied. This acid is used in goods manufacturing, in food processing, in the cleaning industry, in medicine, and as a health supplement. Acetic acid is also a biochemical essential in acetyl group form where it is fundamental to the construction of amino acids and therefore impossible to exist without. Let’s have a look at a few of these acetic acid uses in more detail.
Acetic Acid in Goods Manufacturing
Acetic acid is an important chemical reagent used to produce acetate, adhesives, glues, and synthetic fabrics. Acetic acid is also used in electroplating where a metal coating is deposited onto an object by placing it in a solution that contains a specific metal salt. The solution needs to be conductive and acids that donate hydrogen ions create ideal conditions. Furthermore, electroplating can only occur within a solution and metal salts only dissolve in solutions with a low (acidic) pH value.
Acetic acid is a raw material used for the production of cellulose acetate, acetic anhydride (plastics) and chloroacetic acid used in the production of dyes and pesticides as well as certain drugs.
Acetic Acid in Food Processing
Acetic acid used in food processing to regulate the acidity or alkalinity levels of foods. The Code of Federal Regulations (CFR) categorizes acetic acid as a general-purpose food additive which is safe when used in accordance with good manufacturing practices. In Europe, E-number regulations apply to all food additives. Acetic acid has been given code E260 and is considered a safe ingredient that controls bacterial colonization and can be used without limitation. This is not a new finding. It is said that the ancient Babylonians used vinegar as a food preservative.
Vinegar is used to produce salad dressings, condiments that include mustard, ketchup, and mayonnaise, and in sauces and pickles.
Acetic Acid for Cleaning
Acetic acid has been used as a cleaning product and deodorizer for centuries if not millennia; sponges of vinegar were placed in expensive filigree rings worn by the rich whenever they stepped through filthy and stinking eighteenth-century streets. The deodorizing properties of vinegar have also been taken advantage of for generations. Sailors used vinegar to scrub the decks of the ships they worked and lived on. The principles of microbial control may not have been understood at the time but the fresh-smelling, clean and illness-preventing characteristics of this organic solution were definitely well known.
Adding an alkaline product to acid causes a bubbling, fizzing reaction. Some traditional cleaners believe this effect produces a deeper clean to stable surfaces. For example, scrubbing the back yard with alkaline caustic soda (sodium hydroxide) and then using a vinegar mix on top of this will set off a reaction that certainly looks as if it has a deep-cleaning action; however, this does very little to increase the hygienic effect but rather buffers or works against the alkaline cleaning power of the caustic soda with the acidic properties of vinegar.
Today, many dedicated fans of white vinegar advertise the ecological benefits of using diluted acetic acid to clean bathrooms, wash clothes, remove odors, and make food preparation surfaces both clean and safe. Acetic acid also removes rust and lime scale deposits.
Acetic Acid in Medicine
Acetic acid or vinegar has probably been used in medicine since before the written word. Should you have suffered from an open wound on the island of Kos in the fourth century before Christ, you may have been prescribed a daily vinegar wash by Hippocrates. If you had a sore throat, he might also have asked you to mix honey and vinegar to make Oxymel, an ancient Greek cough medicine; if you had served in Europe during the First World War, you may only have had access to vinegar keep clean and remain free of infection.
Today, acetic acid solutions are used in laboratory blood testing processes as a slide wash. They remove bacterial biofilms in wounds and the digestive system, and have often been used for outer ear infections and so avoid the use of antibiotics. Ingestion of vinegar increases acetate levels in the colon and promotes calcium uptake with lower blood pressure and higher bone density as a result. Studies are looking into the use of acetate as an antitumor medication.
Acetic Acid as a Health Supplement
Acetic acid is a popular health supplement and consumed in the form of vinegar, most commonly apple cider vinegar. When bound to coenzyme A, the acetyl group of acetic acid is central to carbohydrate and fat metabolism.
Much study has been done regarding the link between vinegar consumption and lower blood glucose levels. Where high glycemic index foods are consumed after the ingestion of two to three tablespoons of apple cider vinegar, their glycemic values have been shown to be up to 35% lower. For diabetics, this could mean lower post-prandial blood glucose peaks and better glycemic control and for non-diabetics a lower risk of developing insulin resistance. Substitution of regular cucumber with a pickled cucumber showed a 30% reduction in total meal glycemic index value.
The following image shows the effect of low and high glycemic index (GI) foods on blood glucose levels. High GI foods cause a rapid peak in blood glucose levels that increase insulin production which enables the cells to metabolize glucose. This means that the blood glucose level quickly dips, causing hunger. Low GI foods cause a gentler rise in blood sugar and do not force the pancreas to produce such large quantities of insulin. The result is a gentle curve that remains stable and does not dip, increasing satiety levels after a meal. When a high GI food and low GI food are eaten at the same time they partially cancel each other out, creating a plateau effect. Vinegar is known to have the same effect as a very low GI food.

As already mentioned, acetic acid is also considered to be a weight loss supplement due to its ability to level peak glucose after meals and so helps to increase satiety. It is also suggested that the presence of acetic acid in the diet slows gastric emptying which also helps control some forms of overeating. Furthermore, vinegar seems to discourage fat formation in doses of as little as fifteen milliliters per day.
Quiz
