Definition
The nitrogen cycle refers to the cycle of nitrogen atoms through the living and non-living systems of Earth. The nitrogen cycle is vital for life on Earth. Through the cycle, atmospheric nitrogen is converted to a form which plants can incorporate into new proteins.
Nitrogen Cycle Explained
Nitrogen was originally formed in the hearts of stars through the process of nuclear fusion. When ancient stars exploded, they flung nitrogen-containing gases across the Universe. When the Earth was formed, nitrogen gas was the main ingredient in its atmosphere.
Today, the Earth’s atmosphere is about 78% nitrogen, about 21% oxygen, and about 1% other gases. This is an ideal balance because too much oxygen can actually be toxic to cells. In addition, oxygen is flammable. Nitrogen, on the other hand, is inert and harmless in its gaseous form. However, nitrogen gas is not accessible to plants and animals for use in their cells.
Here we will discuss how nitrogen plays a vital role in the chemistry of life – and how it gets from the atmosphere, into living things, and back again.
Nitrogen Cycle Steps
The basic steps of the nitrogen cycle are illustrated here:
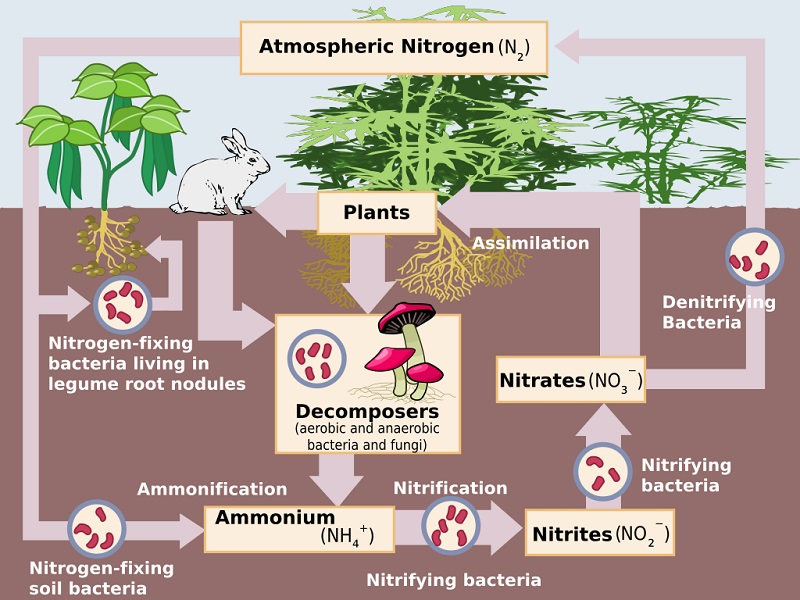
We’ll discuss each part of the process below.
Nitrogen Fixation
In the process of nitrogen fixation, bacteria turn nitrogen gas from the atmosphere into ammonia.
These nitrogen-fixing bacteria, often called “diazotrophs,” have an enzyme called “nitrogenase” which combines nitrogen atoms with hydrogen atoms. Ammonia is a nitrogen compound that can dissolve in water, and is easier for other organisms’ enzymes to interact with.
Interestingly, the enzyme nitrogenase can only function when oxygen isn’t present. As a result, organisms that use it have had to develop oxygen-free compartments in which to perform their nitrogen fixation!
Common examples of such nitrogen-free compartment sare the Rhizobium nodules found in the roots of nitrogen-fixing legume plants. The hard casing of these nodules keeps oxygen out of the pockets where Rhizobium bacteria do their valuable work of converting nitrogen gas into ammonia.
You can see the oxygen-free Rhizobium nodules, visible as big round lumps, on the roots of this cowpea plant:

Nitrification
In nitrification, a host of soil bacteria participate in turning ammonia into nitrate – the form of nitrogen that can be used by plants and animals. This requires two steps, performed by two different types of bacteria.
First, soil bacteria such as Nitrosomonas or Nitrococcus convert ammonia into nitrogen dioxide. Then another type of soil bacterium, called Nitrobacter, adds a third oxygen atom to create nitrate.
These bacteria don’t convert ammonia for plants and animals out of the goodness of their hearts. Rather, they are “chemotrophs” who obtain their energy from volatile chemicals. By metabolizing nitrogen along with oxygen, they obtain energy to power their own life processes.
The process can be thought of as a rough (and much less efficient) analog to the cellular respiration performed by animals, which extract energy from carbon-hydrogen bonds and use oxygen as the electron acceptor, yielding carbon dioxide at the end of the process.
Nitrates – the end product of this vital string of bacterial reactions – can be made artificially, and are the main ingredient in many soil fertilizers. You may actually hear such fertilizer referred to as “nitrate fertilizer.” By pumping the soil full of nitrates, such fertilizers allow plants to grow large quickly, without being dependent on the rate at which nitrogen-fixing bacteria do their jobs!
Interestingly, high-energy environments such as lightning strikes and volcanic eruptions can convert nitrogen gas directly into nitrates – but this doesn’t happen nearly enough to keep modern ecosystems healthy on its own!
Assimilation
In nitrogen assimilation, plants finally consume the nitrates made by soil bacteria and use them to make nucleotides, amino acids, and other vital chemicals for life.
Plants take up nitrates through their roots and use them to make amino acids and nucleic acids from scratch. Animals that eat the plants are then able to use these amino acids and nucleic acids in their own cells.
Ammonification
Now we have moved nitrogen from the atmosphere into the cells of plants and animals.
Because there is so much nitrogen in the atmosphere, it may seem that the process could stop there – but the atmosphere’s supply is not infinite, and keeping nitrogen inside plant and animal cells would eventually result in big changes to our soil, our atmosphere, and our ecosystems!
Fortunately, that’s not what happens. In a robust ecosystem like ours, anywhere that energy has been put into creating an organic chemical, there is another form of life that is waiting to extract that energy by breaking those chemical bonds.
A process called “ammonification” is performed by soil bacteria which decompose dead plants and animals. During the process, these decomposers break down amino acids and nucleic acids into nitrates and ammonia and release those compounds back into the soil.
There, the ammonia may be taken up again by plants and nitrifying bacteria. Alternatively, the ammonia may be converted back into atmospheric nitrogen through the process of denitrification.
Denitrification
In the final step of the nitrogen cycle, anaerobic bacteria can turn nitrates back into nitrogen gas.
This process, like the process of turning nitrogen gas into ammonia, must happen in the absence of oxygen. As such it often occurs deep in the soil, or in wet environments where mud and muck keep oxygen at bay.
In some ecosystems, this denitrification is a valuable process to prevent nitrogen compounds in the soil from building up to dangerous levels.
Why is the Nitrogen Cycle Important?
Nitrogen is an essential ingredient for life as we know it. Its unique chemical bonding properties allow it to create structures such as DNA and RNA nucleotides, and the amino acids from which proteins are built. Without nitrogen, these molecules would not be able to exist.
It’s thought that the first nucleotides and amino acids formed naturally under the volatile conditions of early Earth, where energy sources like lightning strikes could cause nitrogen and other atoms to react and form complex structures
This process might have naturally produced self-replicating organic chemicals – but in order to reproduce and evolve, life needed to figure out how to make these nitrogen compounds on demand.
Today, “nitrogen fixers” are organisms that can turn nitrogen gas from the atmosphere into nitrogen compounds that other organisms can use to produce nucleic acids, amino acids, and more. These nitrogen fixers are such a vital part of the ecosystem that agriculture cannot occur without them.
Ancient peoples learned that if they did not alternate growing nitrogen-consuming crops with nitrogen-fixing crops, their farms would become fallow and unable to support growth. Today, most artificial fertilizers contain life-giving nitrogen compounds as their main ingredient to make the soil more fertile.
The Danger of Too Much Nitrogen
While the importance of nitrogen to plant and animal life might make it sound like there’s no such thing as too much, there are actually some dangers that can arise from putting too many nitrates in the soil.
Like anything else, nitrogen compounds can be toxic in high concentrations. Just like too much oxygen is toxic to air-breathers, plants can suffer harmful effects from nitrogen overdose.
In Humans
Nitrates can also be directly toxic to humans – when consumed in large quantities in food or water, nitrates can increase cancer risks and interfere with blood chemistry, leaving blood unable to properly carry oxygen.
“Blue baby syndrome” is one side effect seen in humans who consume high levels of nitrates in their food or water.
Within Ecosystems
Another acute worry is the danger of throwing ecosystems out of balance. Some organisms can use nitrogen compounds to grow faster than others – and that means that when there’s lots of nitrogen around, these organisms can grow so fast that they cause harm to other organisms.
One concern that has been raised about the use of artificial nitrate fertilizer is that when it gets into rivers, lakes, and even the ocean, it can cause runaway growth of plant life there.
More plant life might sound like a good thing – but not when aquatic plants include algae that can block sun and oxygen from getting to other aquatic organisms, and even produce toxins that make humans and other animals sick!
Nitrate fertilizer in water supplies has been blamed for some blooms of “red tides,” “brown tides,” and Pfiesteria bacteria – all of which produce toxins that can sicken or kill humans and other animals.
The question of how to keep farmlands fertile without using nitrate fertilizers is still being investigated by scientists. It is hoped that someday, sustainable practices using natural or genetically engineered nitrogen-fixing plants may allow farmers to produce high crop yields without adding high concentrations of artificial nitrates to the soil.
Examples of the Nitrogen Cycle
The Story of Thanksgiving
The story of the first Thanksgiving goes that the pilgrims feasted with the Indians to celebrate their first harvest in the New World. But why was this harvest a big enough deal to throw a feast over? And why, exactly, was it important that the Indians and the European settlers ate together?
When the European settlers came to the Americas, they had very little idea of how to survive here. Having worked farms in back in England for generations, the pilgrims assumed that farming here would be very much the same. That turned out not to be the case. The pilgrims had a difficult time growing or finding enough food to last them through the winter.
One of the reasons for that was that there was not much nitrogen in the soil where the pilgrims landed. Their crops were not nitrogen-fixing, and they hadn’t brought any large livestock. This was a major problem, as manure had been a common source of fertilizer in the old world. After trying in frustration to grow crops in the American soil, the Europeans were shown how to solve their problems by the American Indians.
By burying dead fish in their crop fields, the pilgrims restored nitrogen from the fish’s proteins and nucleotides to the ground. As a result, their crops flourished – and the first European settlers learned from the American Indians how to survive in the New World.
The Three Sisters
Some tribes of Native Americans traditionally grow three crops together – corn, beans, and squash.
Often referred to as “the three sisters,” this crop combination is ingenious for several reasons. For one, eating these three plants in combination provides humans with proteins containing all the essential amino acids.
For another, it includes a nitrogen-fixing plant – beans.
The beans contain Rhizobium nodules in their roots, which contain bacteria that can convert atmospheric nitrogen into a form that’s usable by soil bacteria and, ultimately, plants.
Just like burying fish in the fields, growing beans alongside corn and squash assures that the soil does not become too depleted to grow new plants. Even a single crop of corn or squash may grow better alongside nitrogen-fixing beans, as their Rhizobium bacteria nurtures the surrounding soil!
Artificial Fertilizer
Humans first began fertilizing their crops using natural nitrogen-containing substances such, such as dead fish and animal manure. These waste products of animal life contained proteins, amino acids, and nucleotides which soil bacteria and plants could use to grow.
Today, humans have discovered industrial processes which can turn ammonia into nitrates just like those produced by soil bacteria. Plants can use these nitrates directly, and human industry can produce them in large quantities.
Unfortunately, the human impact on the nitrogen cycle makes changes to the environment, which can have unintended consequences. Just as artificial nitrates promote the growth of “good” plants like crops, they can also promote the growth of “bad” plants and algae that produce toxins and outcompete other life forms.
This can be especially problematic when artificial fertilizers are carried by rainwater from farmlands and lawns into rivers and lakes. The result can be the growth of toxic algae that can strangle wetlands and even get into human drinking water.
Quiz
