Definition
A lipid is a fatty, oily, or wax-like compound that is insoluble in water (hydrophobic). It is a combination of glycerol and fatty acids. When mixed in a watery solution, lipids disperse into tiny droplets to produce an emulsion. Lipids are divided into eight categories: glycerolipids, glycerophospholipids, sphingolipids, fatty acyls, sterol lipids, prenol lipids, saccharolipids, and polyketides. A lipid has multiple functions in the human body, from cell membrane construction to energy storage.
Lipid Structure
Lipid molecule structure depends on the type of lipid, yet all contain the basic component of the fatty acid. A fatty acid is a straight chain of four to twenty-four carbon atoms with hydrogen atoms running along the carbon chain and a carboxyl group (-COOH) at one end. The presence of the carboxyl group provides the acid component.
How the carbon atoms link together determines whether a fatty acid is saturated or unsaturated. Single, low-reactivity bonds produce saturated fatty acids. Where one or more double or triple bonds are present between two carbon atoms, the fatty acid is unsaturated and has a higher level of reactivity with its surroundings. Double or triple bondson a single carbon molecule make the chain bend into a cis conformation.
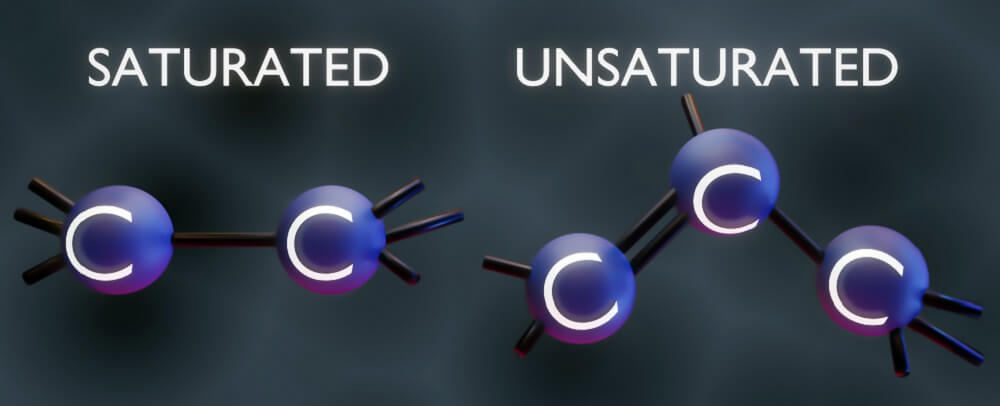
Cis and trans are forms of isomers. Isomers are molecules with the same number of atoms of each element; however, these atoms can be arranged differently. Varying arrangements make molecules behave in different ways. For example, cis isomers are denser, more soluble in certain solvents, and have higher boiling and lower melting points.
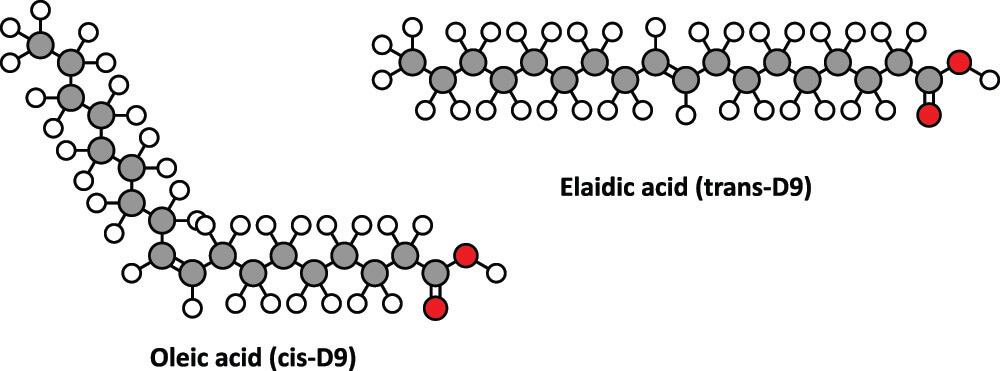
Cis means adjacent and describes how bonds occur on the same side of an atom. This makes the molecule denser to one side. A trans-isomer, where trans means across, has equal bonds on both sides of an atom.
When a cis-chain is denser on one side, it will bend. This can provide a more compact structure, as in the case of a cis fatty acid. Such characteristics and the distribution of bound molecules give rise to the many different lipid types.
Fatty acids rarely exist as single molecules and are most commonly found in the form of triglycerides (glycerolipids). A triglyceride is the combination of three (tri) fatty acids and a single glycerol molecule. Glycerol is an alcohol and termed the “sweet principle of fat” when first described in 1783.
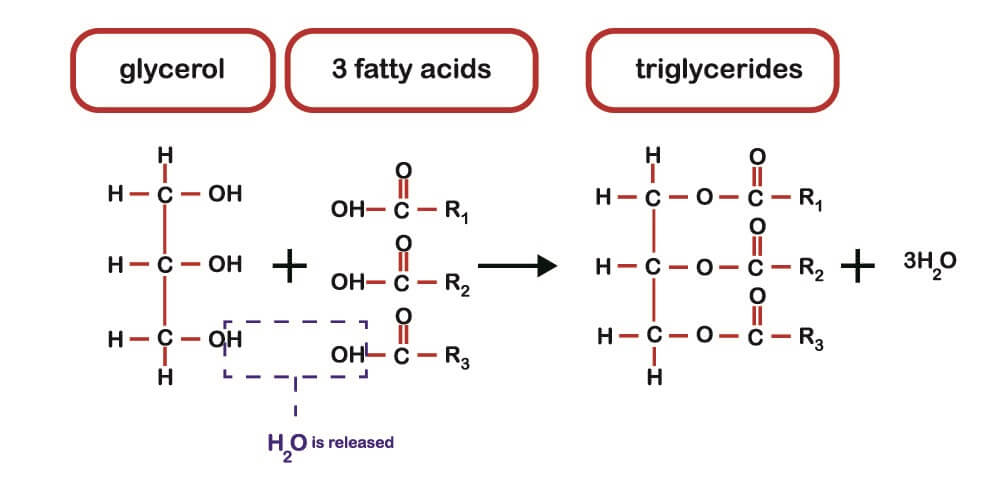
Triglycerides are formed by the attachment of three fatty acids to a glycerol molecule. Phospholipids attach in the same way but only contain two fatty acids. The bonds are ester bonds formed by a condensation reaction (see above image). Where three fatty acids bind to glycerol, three molecules of water are released. In the case of phospholipid formation, two molecules of water are released.
A triglyceride has three ester groups and is sometimes called a tri-ester molecule. Triglycerides formed from cis-isomers are commonly liquid (oils) at room temperature; those with trans-isomers are usually solid. Most trans-fats are considered unhealthy.
The condensation reaction can be reversed by a hydrolysis reaction. When the enzyme lipase is present, reversal occurs much more rapidly. Adding water breaks the ester bonds and produces separate glycerol and fatty acid molecules.

Lipid Synthesis
Lipid synthesis occurs when an animal consumes more carbohydrate than is required for its energy needs. Sugars are converted into lipid droplets that are stored in adipose cells (fat cells).
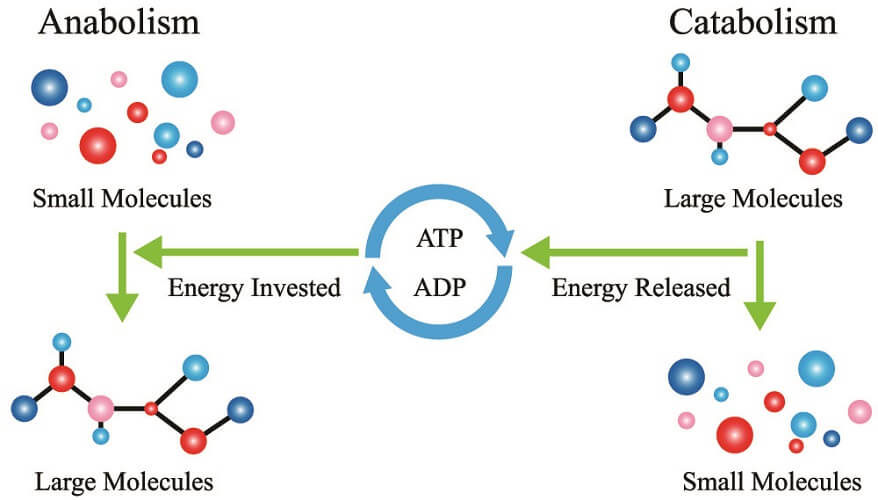
The topic of lipid metabolism tends to be confusing. It is important to understand that two processes exist – the first is energy production in the mitochondria when glucose levels are low; the second is the production of lipid molecules (lipid synthesis) used in various functions throughout the body.
Lipid Catabolism
Catabolic lipid reactions produce energy from stored triglycerides. These are energy-producing processes. Fatty acids must first be separated from glycerol inside lipid cells. Once free, they travel via the bloodstream to cells that contain mitochondria. As fatty acids prefer not to exist as singular molecules in the body, they must bind to a protein in the blood (albumin). Albumin is a serum fatty acid transporter.
Once in the cell cytoplasm, enzymes cause the fatty acids to become fatty acyls. A fatty acyl can attach to a coenzyme A (CoA) molecule to form acyl-CoA. This is a fatty acid chain bound to one coenzyme A – a reaction called CoA acetylation. Only in this form can lipids enter the mitochondria.
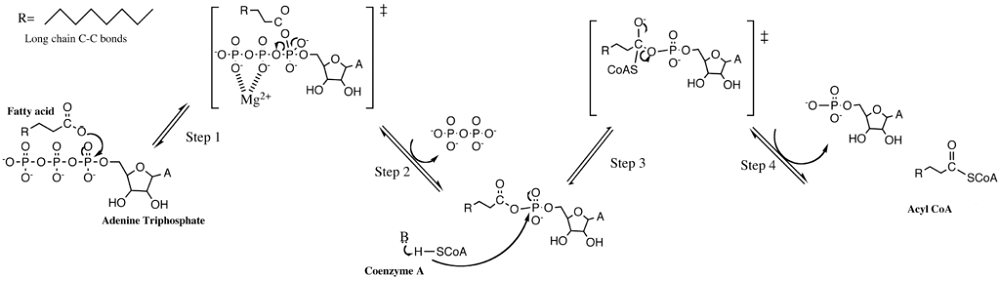
It is now time for a process called beta-oxidation. This is the precursor step to the Krebs cycle where intracellular energy is produced. Remember that beta oxidation is a step in the production of energy from fats when glucose levels are low. Glucose metabolism is a much more efficient process and, therefore, preferable.
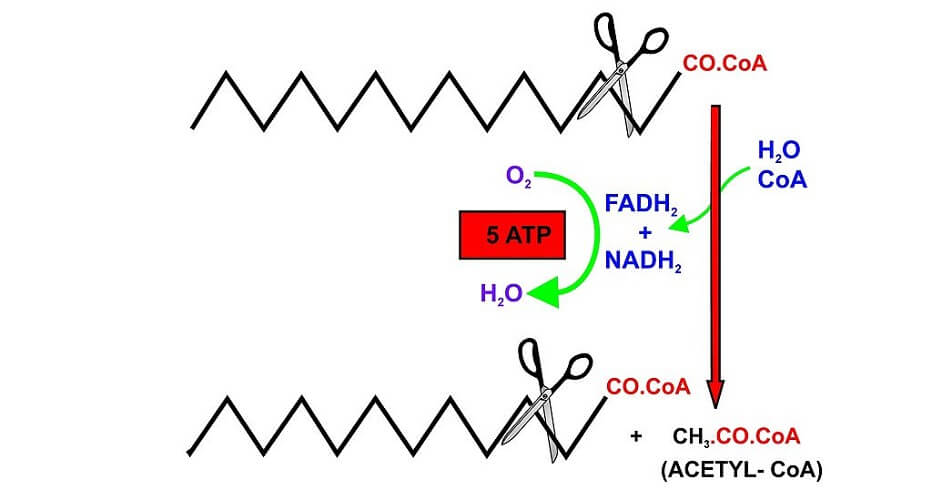
Intracellular energy production is extremely dependent on the availability of acetyl-CoA. During beta-oxidation, acyl-CoA molecules are shortened until only very short fatty acid chains are left attached to the coenzyme A molecule. Only a two (sometimes one) carbon acetyl-CoA molecule can be directly used in the citric acid cycle to produce cellular energy.
Lipid Biosynthesis
Lipid anabolism describes the production of complex lipid molecules from simple ones using energy in the form of adenosine triphosphate (the energy source produced in the citric acid cycle). As many different types of lipid exist, different molecules are formed from basic fatty acids.
It is acetyl-CoA that starts the process. However, the necessary enzymes and even location differ from that of the catabolic process. This prevents important sources of acetyl-CoA from being depleted in the mitochondria. Without lipid production, our health is negatively affected, but a cell’s inability to produce energy is significantly more damaging.
Fatty acid synthases (FAS) are enzyme complexes that help to build fatty acid chains onto acetyl-CoA. These enzymes are present in huge amounts in fat tissue and the liver.

In the endoplasmic reticulum (ER), the fatty acyl chains of acetyl-CoA are lengthened two carbons at a time. When the CoA is released and replaced with glycerol, fatty acids are the result. The difference between fatty acyl and fatty acid is the backbone molecule of CoA or glycerol, respectively.
All biosynthesized (produced inside the body) fatty acids are trans-fatty acids. Desaturase enzymes help to form cis-isomers at a later point within the ER. Humans are unable to make very long unsaturated fatty acid chains (polyunsaturates) and these essential lipids must be obtained from the diet.

Which lipids are produced depends on instructions in our DNA and cell signaling processes. Different lipid types and functions are discussed later on.
Lipid Peroxidation
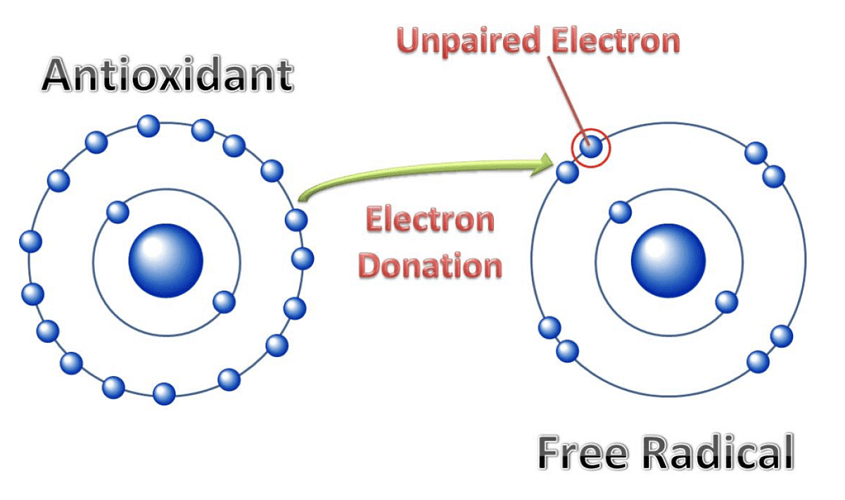
Lipid peroxidation is a potentially damaging mechanism if left to continue without regulation. When electrons are taken from cell membrane lipids, peroxidation occurs. Reactive oxygen species (ROS) react to produce an unstable fatty acid radical (a fatty acid missing electrons in its valence shell) and water.
To become more stable as is required under the octet rule, the fatty acid radical reacts with oxygen. Even so, even these radicals are unstable and they continue to react – usually with each other. This chain of events only halts when the molecule becomes stable (non-radical). Lipid peroxidation is a snowball effect that can only be halted by antioxidants.
When the process is not stopped, damage occurs in structures that contain lipids. This is most commonly the membranes of our cells. Lipid peroxidation is known to play a role in cancer development.
Lipid Functions in the Human Body
When studying lipid functions in the human body, it is easier to look at the different categories as mentioned in the lipid definition at the top of this page. Lipid categories are described according to the Lipid Metabolites and Pathways Strategy (LIPID MAPS) Lipidomics Gateway classification system.
Fatty Acyl Function
As we have already seen, fatty acyls attached to CoA are important components of energy production (lipid catabolism) and lipid synthesis (lipid anabolism). Fatty acyls are, therefore, building blocks for every lipid category in all living organisms, including plants.
One example is the use of fatty acyls in the synthesis of acylcarnitine that transports long-chain fatty acids into the mitochondria. This transport system is called the carnitine shuttle.

Glycerolipid Function
Glycerolipid structure has already been discussed as this describes the most common lipid in the human body – the triglyceride. Most of our body fat is stored in the form of oily triglyceride droplets.
It is also possible for a glycerolipid to be a monoglyceride or a diglyceride – one or two fatty-acid chains attached to a glycerol molecule, respectively.
Glyceroglycolipids are a subclass of glycerolipids where other simple sugars, such as galactose, attach to the glycerol backbone.
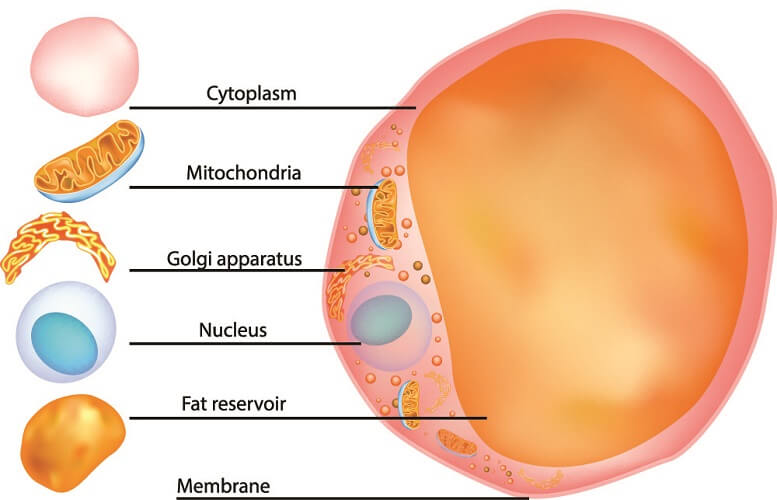
Glycerophospholipid Function
Glycerophospholipids are the most commonly-quoted lipid examples in textbooks as these form the lipid bilayer of cell membranes.
The phospholipid bilayer produces a protective, partially-permeable membrane around the cell cytoplasm. Not only fatty acids and glycerol but also phosphoric acid determine the characteristics of this lipid type.
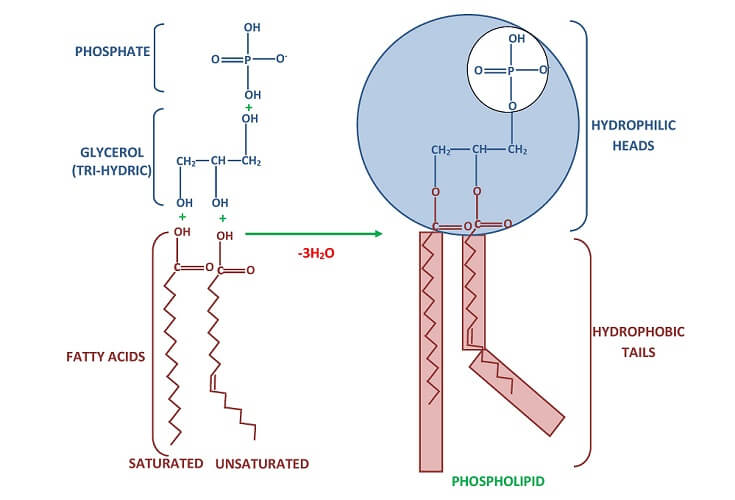
One glycerophospholipid example is lecithin (phosphatidylcholine) that is composed of phosphoric acid, choline, glycerol, and two fatty acids. Different forms of lecithin exist due to differences in isomer shape, chain length, and degrees of fatty-acid saturation.
Lecithin is used in cooking fats and as a health supplement, although little research has been done on its physiological benefits.
Sphingolipid Function
Sphingolipids do not always contain glycerol. Their defining characteristic is the presence of amino acids (sphingoid base backbone) and a long-chain fatty acyl-CoA.
This molecule might also feature proteins, phosphoric acid, and sugars. Sphingolipids have been shown to affect the structure of cell membranes and define how these membranes interact with surrounding fluids and cells; they also help to form phagosome vesicles.
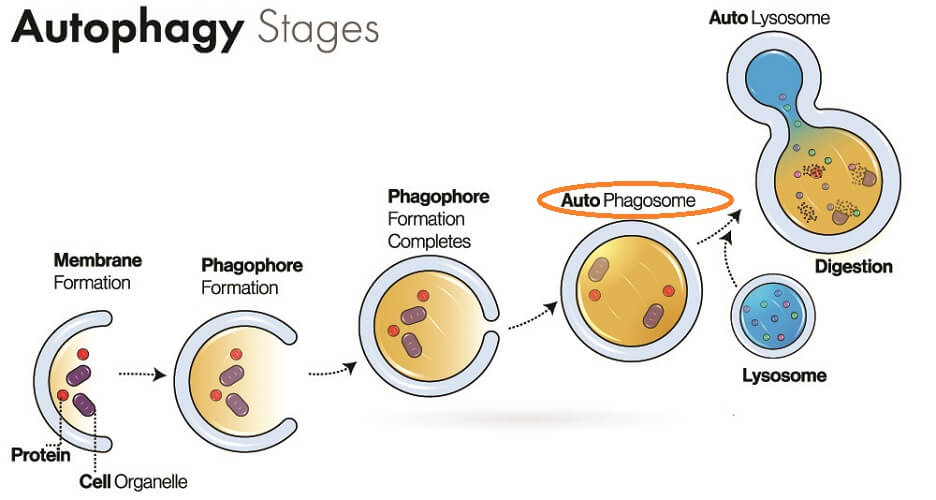
One group of sphingolipids is the ceramides, often found in skincare products. They increase cell membrane strength, even in the dermis.
Lipidomics research has shown that acne often shows alterations in skin-surface lipids: higher levels of glycerophospholipids, fatty acyls, and sterol lipids, and lower levels of prenol lipids and saccharolipids. A study also reported shorter ceramide chain lengths in men with acne.
Saccharolipid Function
When glycerol is replaced with sugars such as glucosamine, important signaling molecules are the result. The main topic of saccharolipid research today is the glycan. Kdo2-lipid A is an acylaminosugar glycan of the saccharolipid class. It functions within our immune system and is important for efficient nerve-signal transduction.
Glycans are saccharide units linked to lipid or protein scaffolds. They cover the surface of all cells. Even though pathogens can recognize and attach to them, glycans are able to change shape and prevent further attacks. They either obstruct or encourage cell membrane interactions.
Lipid glycosylation – the attachment of lipid to sugar – is a secondary protein-processing stage inside the cell that determines the stability and function of proteins. By adding fatty acids, different functioning proteins are formed.
Research carried out in reaction to the COVID-19 pandemic has shown that glycans are important to the coronavirus envelope and its binding receptor. Viral glycan-based receptors feature lipid-bound sialic acid. When our cells bind to the sialic acids of the spike protein, viral infection rates increase in the body. This also increases the transmission rate from animals to humans.

Polyketide Function
Polyketides are becoming popular anti-microbial and even anti-cancer agents; many of our antibiotics are part of this lipid classification group.
Natural polyketides are formed from acyl-CoA but seem only to occur in marine creatures such as sea slugs and plants, fungi, and bacteria. Humans do not appear to produce polyketides even though they are present in the body. Instead, they are produced by the bacteria inside us.

Polyketides are important component of a broad range of medicines. One example of a polyketide lipid-based drug is the macrolide antibiotic erythromycin, used to treat a variety of bacterial infections.
Sterol Lipid Function
Sterol lipids include steroid hormones with essential signaling roles. All of our adrenal and sex hormones are sterol lipids. Steroids are a subcategory of prenol lipids but are so structurally distinct they are given their own category.
Is cholesterol a lipid? Certainly. It is a sterol lipid and the precursor of all steroid hormones. As with all sterol lipid structures, cholesterol is formed from hydrocarbon rings.
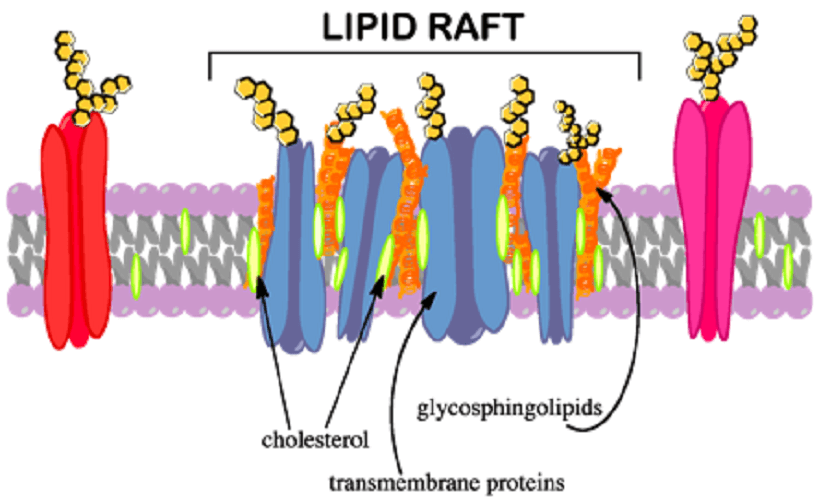
Cholesterol is an extremely important lipid found in cell membranes, the brain, bile, and several vitamins and hormones. It plays a role in intracellular transport and signaling. It is also found in high quantitites, together with sphingolipids, in cell membrane lipid rafts (areas with lots of lipids). Lipid rafts are associated with signal transduction, vesicular transport, organization of the cytoskeleton, and pathogen entry.
While higher cholesterol levels used to be seen as a health risk, blood lipid panel testing is now more likely to look at the presence of elevated triglycerides to predict cardiovascular disease.
A lipid profile screens for the presence of triglycerides and cholesterol in blood serum. It also measures the ratio of low to high-density lipoproteins – LDL and HDL. The protein parts of these two molecules are carriers for the lipid cholesterol.
Prenol Lipid Function
Prenol lipids or terpenoids make up a group that includes quinones and certain vitamins. These lipids are the result of terpene units that are themselves formed from two or more isoprene molecules. Isoprene is the most common synthesized hydrocarbon in the human body. We make so much of it, it can be detected in our breath. Fatty acids bound to terpene units form prenol lipids.
The fat-soluble vitamins A, D, E, and K are all prenol lipids. The essential oils we obtain from plants are also isoprene-based lipids. Another group of prenol lipids, the quinones, are essential for intracellular aerobic metabolism in living organisms. They are known to protect again reactive oxygen species (ROS) and, because of this, are antioxidants. That is why essential oils are used to treat a range of medical conditions (aromatherapy).