Hypertonic Solution Definition
A hypertonic solution contains a higher concentration of solutes compared to another solution. The opposite solution with a lower concentration is known as the hypotonic solution. Scientists must describe cell contents compared to the environment. If a cell is placed in a hypertonic solution, the cell is considered hypotonic.
Hypertonic Solution Overview
If the cytosol of the cell is a hypertonic solution, it means the environment is hypotonic, or more weakly concentrated. This is of great importance because solutes and water tend to flow or diffuse along their gradients. Two solutions mixed together will eventually become a single solution. If the solutions are separated by a permeable membrane that only allows water through, the solutions will become isotonic as the water moves between the two solutions. Isotonic solutions have equal concentrations, although they may have different volumes.
This movement of ions and water is extremely important to cells. Cells use ion gradients for a number of purposes. For example, plant cells use a hypertonic solution within their central vacuole to help draw water into the vacuole. This expands the chamber and allows plants to create turgor pressure in their cells. Animal cells, especially nerve cells, rely on a hypertonic solution and the ions in it to create an action potential or nerve signal. The electrical activity of these cells relies on the positive and negative charges of the ions in the hypertonic solution.
Hypertonic Solution Examples
Human Kidney
To regulate the amount of water in the body, the human brain has special proteins called osmoreceptors, which can measure the osmolarity of the environment surrounding the cell. If the environment becomes a highly hypertonic solution, it is because there is not enough water in the blood to dilute the solutes. The hypothalamus releases hormones while increasing the permeability of membranes in the kidney. The kidney resorbs the water that would have been excreted and adds it back to the bloodstream. The blood becomes more isotonic compared to the cells, and normal processes can continue.
Sea Turtle Osmoregulation
Compared to fresh water, salt water is a hypertonic solution. This means that for cells to function, they must contain a cytosol that is a more hypertonic solution than salt water. Sea turtles, for example, live in a much more hypertonic solution compared to freshwater turtles. If you put a freshwater turtle in seawater, the hypertonic seawater will dehydrate the turtle. Instead of being hydrated by the water, the solute-dense ocean water will pull water from the body to balance the difference in osmolarity.
To overcome this obstacle, sea turtles and other sea animals have developed unique pathways to remove excess salts. The salts move from the digestive tract into the bloodstream. When they reach the salt gland, they are removed. This creates an internal environment that is higher in solutes, but one that doesn’t lose excess amounts of water to the environment.
Plants in Hypertonic Solution
Generally, plants prefer to live in hypotonic environments. In a hypotonic environment, water easily floods plant cells and they can remain turgid, or rigid, due to pressures exerted on their cell walls by the influx of water. The plants use this water potential to give their bodies structure and move water from the roots to the top of the plant. However, many plants have adapted to live in hypertonic environments. Marshes by the sea, mangrove swamps, and other brackish waters contain a much higher salt content than fresh water. The soil becomes saturated with these salts, creating a much higher solute concentration in the soil.
Most plants would shrivel up if they were transplanted to this habitat, but a special group of plants known as Halophytes has evolved to overcome this obstacle. By increasing the osmolarity of their roots, the plants are able to change from a hypotonic environment inside the cell compared to the environment, to a hypertonic solution in the cytosol. This lowers the water potential of the root cells and allows water to enter the cells. The cells either store the excess salts in the roots or transport the salts to the leaves, where they can be excreted out of glands.
A Cell in Hypertonic Solution
The plasma membrane that surrounds cells is a special permeable membrane that separates the contents of the cell from the environment. The plasma membrane is embedded with special membrane transport proteins that help transport solutes across. It also has special protein channels called aquaporins that allow water to flow freely across the membrane. The cell must use energy to actively move solutes into and out of the cell. Too many solutes and the cytosol will become a hypertonic solution compared to the environment. Cells without cell walls can burst in this condition.
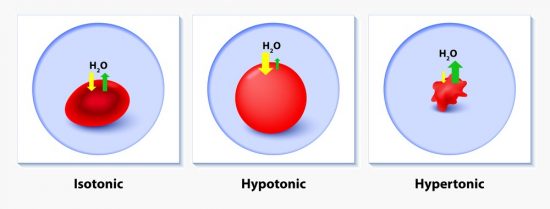
Too few solutes in the environment will become the hypertonic solution. In this case, the opposite will happen, as water moves out of the cell. Water moves against the concentration gradient of solutes, moving from areas of low solute concentration to areas of high solute concentration. In another sense, water moves with the water concentration gradient, from areas of high water concentration to areas of low water concentration.
Organisms that regulate the osmolarity of their cells are known as osmoregulators. Typically, cells try to maintain their cytoplasm as a hypertonic solution compared to the environment. While this does pose certain structural problems, it allows water to flow freely through the cell, and participate in many of the necessary reactions. If cells were hypotonic, they would eventually lose most of their water to the environment. Other organisms, osmoconformers, have the same osmolarity as the environment, although the exact solutes may be different. This ensures that they neither lose nor gain lots of water.
Quiz
