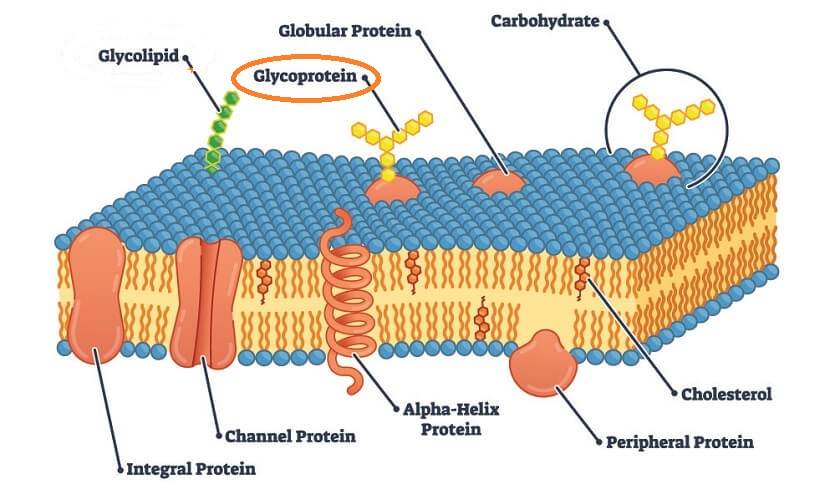
Definition
A glycoprotein is part of an extremely diverse group of linked amino acid and carbohydrate chains. Glycoproteins are found throughout nature and have a similarly diverse range of functions. They are one of two glycoconjugates – the other group is composed of glycolipids. Glycoprotein examples include fibrillins, mucins, tumor necrosis factor, and membrane glycoproteins on body cells, bacteria, and viruses.
Glycoprotein Structure
Glycoprotein structure is described in its name – a sugar portion (glyco) attached to a protein. The two components attach by way of a covalent bond.
Most scientists refer to the carbohydrate (sugar) part of a glycoprotein structure as a glycan. Glycans are oligosaccharides and can form between one and eighty percent of a glycoprotein structure. Before binding to a protein, this sugar molecule is called a glycosyl group.
The removal of a hydroxyl group (-OH) from a monosaccharide in a polysaccharide chain forms a glycosyl group with a vacant spot that makes it unstable. To date, we know of thirteen monosaccharides that attach to eight different amino acids. These attachments are necessary to make an unstable monosaccharide stable again. The hydroxyl group of the associated amino acid replaces the lost –OH of the glycosyl group.
Adding glycosyl to a peptide chain, protein, or lipid is called glycosylation. More than half of the proteins in the human body are glycosylated. In eukaryotic cells, glycosylation occurs in the endoplasmic reticulum and Golgi apparatus.
There are two primary glycosylation processes; the first process binds a small sugar molecule (oligosaccharide) to the asparagine amino acid of a polypeptide chain. The second binds a glycan to either serine (N-linked glycosylation) in the endoplasmic reticulum or threonine (O-linked glycosylation) in the Golgi apparatus.
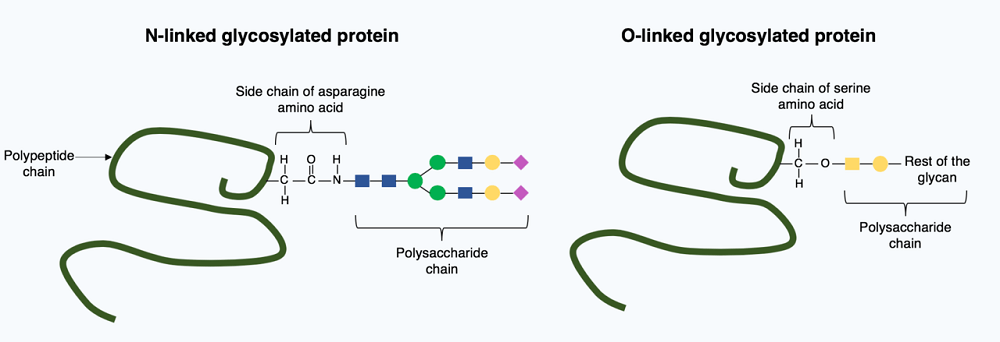
Glycosylation is a complex process and requires the intervention of several enzymes. When no enzymes are used to link a sugar molecule to a protein (or lipid) the process is called glycation. It is also possible to break the bond between sugar and protein through the deglycosylation process. However, while glycans help polypeptide chains to fold into functioning proteins, deglycosylation of a fully-formed protein will not cause it to unfold or stop functioning.
Glycoprotein Function
Glycoprotein function occurs throughout the body; this group is both widespread and diverse and is integral to many biochemical processes.
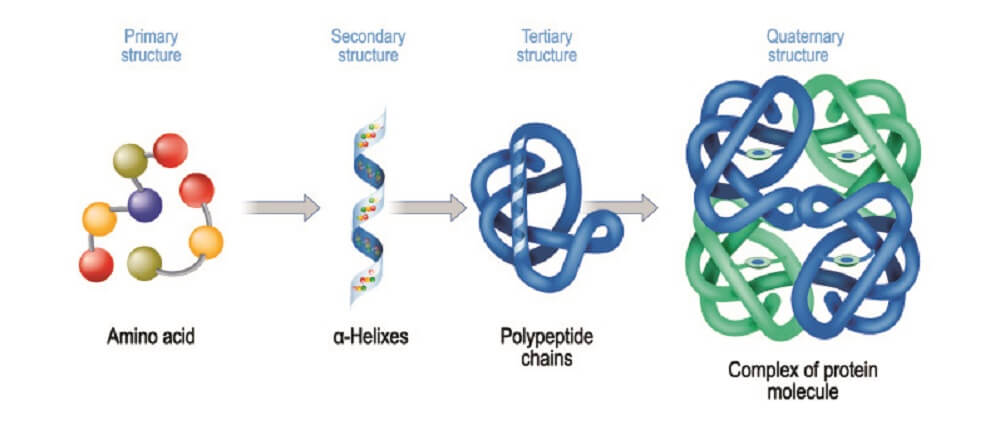
Once amino acid chains have been produced through intracellular protein synthesis, they must undergo further reactions to become biologically functional. As we have just seen, adding glycosyl to certain inactive amino acid chains (polypeptides) produces functional, folded glycoproteins.
As glycans are hydrophilic, the formation of a glycoprotein can make the molecule more soluble. The covalent bond between the polypeptide chain and glycan also forms a highly-stable molecule that prevents peptide bonds from breaking down during a process called proteolysis. Glycoproteins can withstand higher or lower temperatures than simple proteins, for example.
Probably the most commonly-quoted glycan function is this group’s ability to make proteins fold into their final, functional forms. Without the sugar part, many polypeptide chains are unable to form folded protein structures.
Glycoproteins are excreted from inside the cells that express their genes and pushed to the surface. They can be secreted to other parts of the body or sit on the cell surface as membrane glycoproteins. This makes glycoproteins integral to many biological processes: enzymes and hormone formation, the blood clotting, cascade, transfer molecules, signaling proteins, immune system contributors, lubricants, and as structural components. Some antarctic and arctic fish have specific glycoproteins that lower the freezing point of their blood serum, for example. These are often referred to as antifreeze glycoproteins.

Proteoglycan vs Glycoprotein
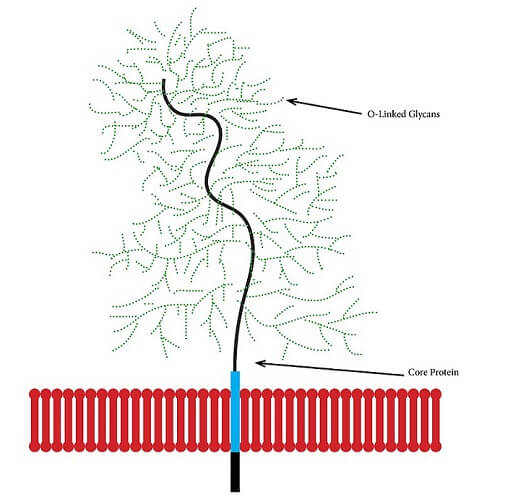
A proteoglycan is a subtype of glycoprotein found in cell membranes within mucus and connective tissue; it is sometimes called a mucoprotein. A mucoprotein is composed of core proteins covalently-bonded to glycosaminoglycans (GAGs).
A GAG is a chain of repeating disaccharide (simple sugar) molecules. The structure below depicts part of the cartilage matrix, an area where glycans are extremely prolific.
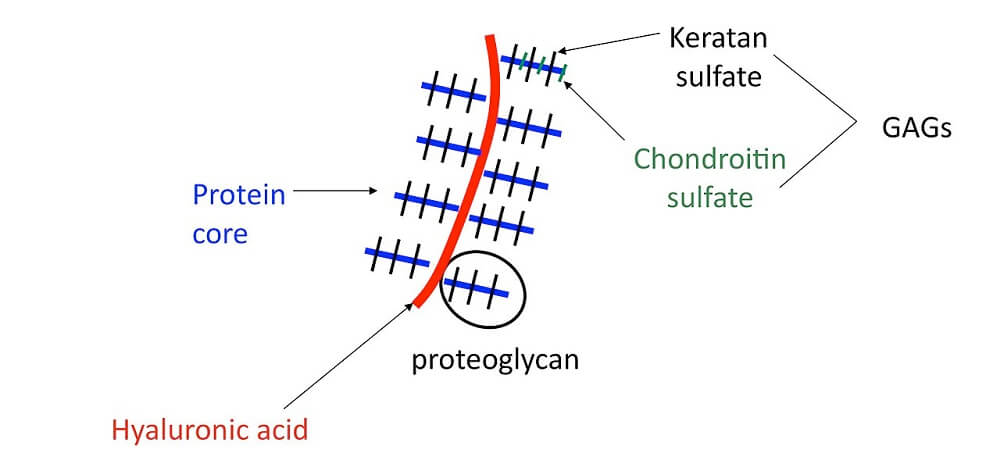
Any compound containing sugars (carbohydrates) attached to other chemicals is classed within the vast glycoconjugate group. Glycoconjugates can be further separated into glycoproteins, glycopeptides, peptidoglycans, glycolipids, and lipopolysaccharides. This article does not include carbohydrate lipid conjugates. As we have seen in proteoglycan vs glycoprotein, proteoglycans are a subcategory of glycoproteins.
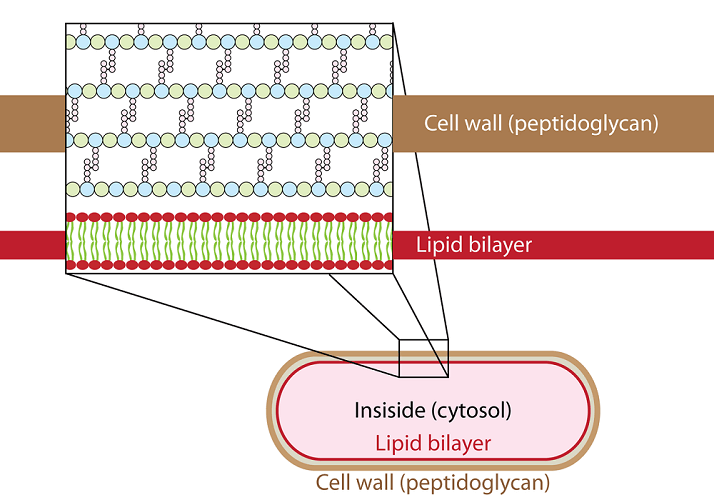
Peptidoglycans or mureins are found only in bacteria. These conjugated carbohydrates are located in the cell wall and do not contain proteins but very short amino acid chains (oligopeptides). They help the bacteria to keep its shape and also aid passive transport (osmosis) through the bacterial cell wall.
Glycoprotein Examples
It would be impossible to list every example of glycoprotein function. There are distinct differences in the glycoproteins of species; those found in the rat can be very different from those of the mouse. This article looks at just a handful of the most important human glycoproteins.
Serum Glycoprotein
Hundreds of glycoproteins exist in our blood plasma and form what is referred to as the serum glycoproteome. Many individual glycoproteins can be measured with a simple blood test. Examples of serum glycoproteins are cartilage oligomeric matrix protein (COMP) and hepatocyte growth factor activator (HGFA). These are inflammatory markers linked to arthritis and fibrosis respectively.
Too high or low quantities of specific glycoproteins in the blood can point to genetic mutations, breakdown of those tissues or the systems they are meant to play a role in, and the presence of disease.

Zona Pellucida Glycoprotein
Without glycoproteins, we cannot reproduce. The zona pellucida that surrounds human egg cells regulates how and whether a sperm cell can bind to and move into released, unfertilized eggs.
The human zona pellucida has four glycoproteins (ZP1 through to 4) and these are known to contribute to egg/sperm interaction. Recent studies show that infertile females often have a mutation in the gene that encodes for ZP1.
Cartilage Glycoprotein
Research tells us that an excessively high level of the human cartilage glycoprotein YKL-40 is responsible for faulty cartilage remodeling in patients with osteoarthritis. Glycoproteins in structural tissues help the main components – in the case of of cartilage, collagen and fibrinogen – form a strong, fibrous network and hold more water. The increased affinity for proteins in water due to the presence of glycans also means cells can travel more easily through this avascular tissue.
Mucin-Type Glycoprotein
Nearly all mucins are the result of O-linked oligosaccharides and can be found in the airway, digestive system, sweat glands, breasts, and even cancer cells. Mucin is a natural barrier that protects the environment surrounding the tissues and cells it is formed by.
A mucin polymer is either attached to the cell membrane or part of the gel-like mucus itself. Glycoproteins play a major role in innate immunity, hydration, lubrication, and enzyme activity – most mucus contains antimicrobial enzymes.

Glycoprotein Hormones
Follicle-stimulating hormone, luteinizing hormone, and thyroid-stimulating hormone are just three hormone glycoprotein examples. We know that the addition of sugar to a protein affects that molecule’s half-life – the longer the glycan chain, the longer the half-life. A half-life is the time it takes half of a biological substance to metabolize and be eliminated.
Immune Glycoprotein
Our immune system can recognize surface membrane glycoproteins by way of receptors such as galectins, C-type lectins, and SIGLECs.
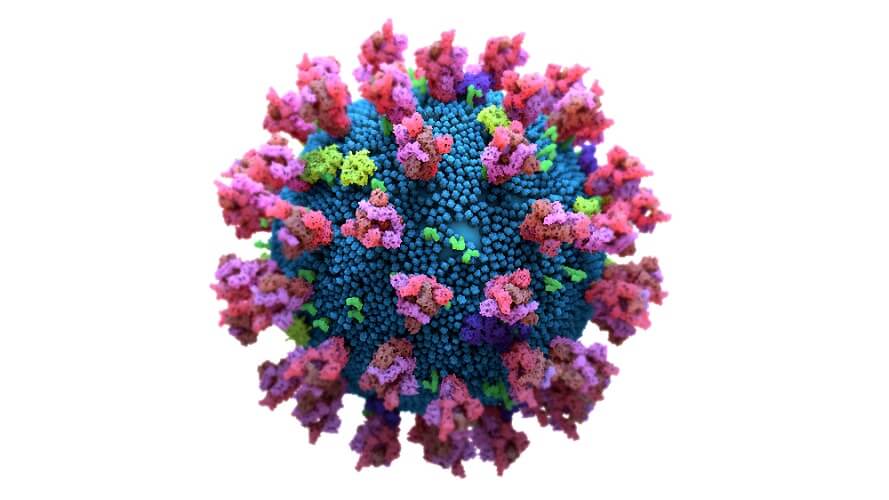
Any immune cell that expresses such receptors can bind to certain secreted glycoproteins on the membranes of other cells. The coronavirus spike glycoprotein that sits on the virus surface increases production of three immune receptors: SIGLEC5, SIGLEC9, and SIGLEC11. While these provide an immediate immune response, they also focus the body’s energy on the short-term immune response and so lengthen the time between the generic primary and specific secondary immune response. This means a lack of coronavirus-specific antibodies.
The pause is the result of spike glycans of SARS-CoV-2 that contain sialic acid; sialic acid is quickly recognized by the three mentioned SIGLEC receptors. The delay in production of virus-specific antibodies gives this terrible virus valuable time to reproduce.
A further example is Beta-2 Glycoprotein 1 antibodies or autoimmunoglobulins that cause the body to attack its own cells. In this case, the glycoprotein antibody affects how well the blood clots. It is an antiphospholipid immunoglobulin that wrongly sees glycoprotein in cell membrane structures as harmful. The presence of membrane beta-2 glycoprotein immunoglobulin (B2GPI) is associated with the spontaneous development of blood clots.
Glycoprotein IIb IIIa Inhibitors
Glycoprotein IIb IIIa inhibitors are also associated with the blood clotting response. When these two glycoproteins block glycoprotein IIb and IIIa receptors, platelets cannot clump together to form a clot. Medications based on this principle are used during certain types of coronary artery procedures to prevent blood clot formation.
When such inhibitory glycoproteins are injected into the blood, platelets are unable to aggregate. Aggregation (clotting) depends on the presence of two important glycoprotein receptor subunits – α and β. Both are found on the platelet cell membrane and, when platelets are activated, allow these cells to stick to each other.
When glycoprotein IIb and IIIa inhibitors are administered, these bind to the receptors and stop natural adherence substances (fibrinogen and von Willebrand factor) from binding and initiating the blood coagulation cascade.