Definition
Thymus- derived lymphocytes (commonly known as T cells) are cells in the adaptive immune system that attack invading pathogens and infected host cells depending on the T cell type activated. There are two major types of T cells: CD8+ and CD4+. CD8+ T lymphocytes are activated into cytotoxic T cells when antigens are presented to them on class I MHC proteins. Meanwhile CD4+ T lymphocytes are activated into helper T cells when antigens are presented on class II MHC proteins. Cytotoxic T cells kill the body’s own cells when infected with a virus or cancer, while helper T cells assist the immune response when the body is attacked by foreign pathogens (such as bacteria). Helper T cells accomplish this by using TH1 cells to recruit phagocytic cells to the pathogen, while TH2 cells aid antibody production by activating B cells.
T cells can be targeted by viruses such as HIV, where infection reduces available T cells in the body. This allows the body to succumb to new pathogens, as the immune system can no longer properly fight the threat.
The Immune System
The immune system is a series of processes utilized to protect the body from infections and diseases. It is compromised of the innate and adaptive systems. The innate system is the initial response when a pathogen first enters the body. It is both quick and non-specific. Meanwhile, the adaptive system is the following immune response. It is much slower initially, but “adapts” to be specific to the threat present. Both the innate and adaptive immune responses utilize white blood cells, known as leukocytes. However, each immune response optimizes specific leukocyte types. The leukocytes commonly found in the innate and adaptive immune systems can be found in the diagram below.
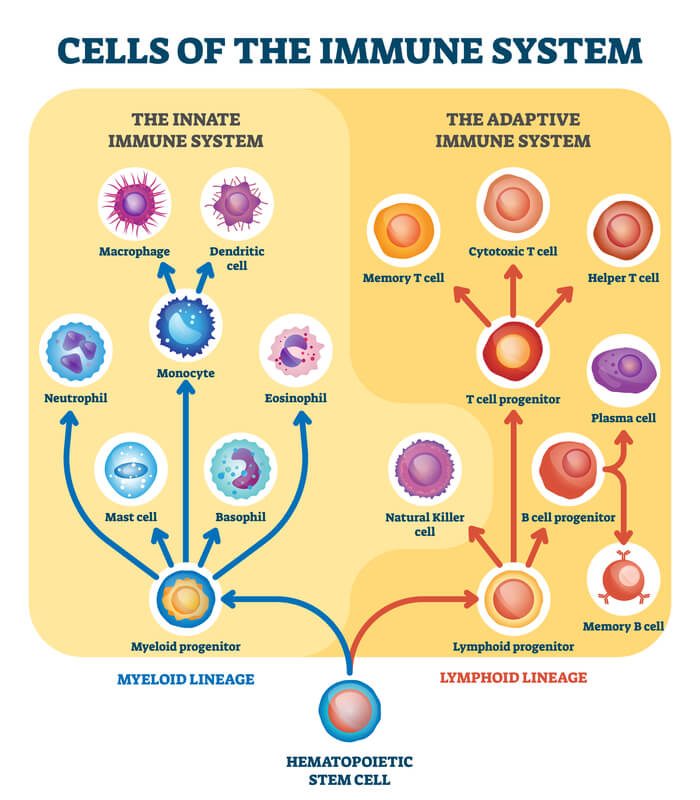
The innate immune system commonly recruits basophils, masts cells, eosinophils, neutrophils, and monocytes. Monocytes can then be differentiated to become macrophages and dendritic cells. Meanwhile, the adaptive immune system commonly recruits T cells, B cells, all of their variations (which will be defined throughout this article), and natural killer cells.
The Innate Immune System
The innate immune system is the first immune response at wounds and pathogen entry points. It is a quick and generic response that is optimized to target any pathogen. Generally, this is done as the immune cells in the host’s body recognize specific proteins and/ or sugars on the membranes of the invading pathogens. Specifically, the host cells detect that these proteins/ sugars are foreign and therefore must be targeted by the immune system. In the case of pathogenic bacteria, the lipopolysaccharides (a sugar molecule) on the bacteria’s membrane are recognized by toll-like receptors, which are proteins on the host’s leukocyte membranes. Lipopolysaccharides are present on all gram- negative bacteria and do not occur in humans. They are thus a reliable target to alert the body of foreign invaders and activate the immune response.
(Note: There are multiple toll- like receptors in the human body, each of which responds to different stimuli. Toll- like receptor 4 recognizes lipopolysaccharides from gram- negative bacteria, while toll- like receptor 2 recognizes zymosan- a molecule in fungi- and toll- like receptor 7 binds to single- stranded viral RNA.)
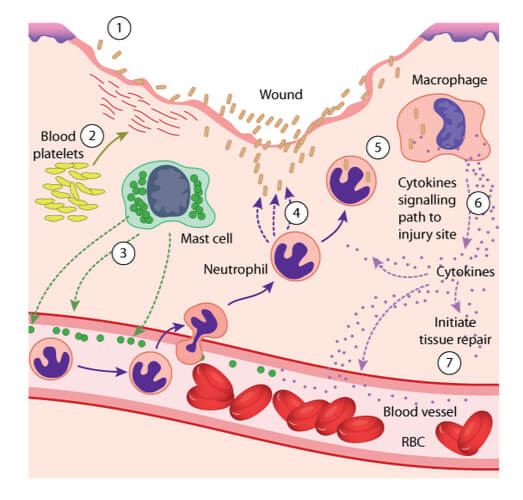
Activation of the immune response begins with the inflammatory response. Initially in this response, macrophages are recruited to the wound site. Here, they secret chemokines, which are molecules that form a chemical gradient to recruit additional immune cells to the wound site. These additional cells can include mast cells, which release histamine to dilate blood vessels further away from the wound. The dilated vessel and chemokine combination then attracts neutrophils, which exit the dilated vessels and gravitate towards the wound site. The neutrophils “eat” the foreign materials through a process known as phagocytosis. During phagocytosis, an enzyme found inside the neutrophil- known as lysozyme- kills the invading pathogen.
The immune response does not end with phagocytosis. Additional monocytes are then recruited to the wound, where they can be differentiated into macrophages. Macrophages also have the ability to release chemokines, in addition to cytokines. Cytokines are immune markers that can cause several effects throughout the body, including fever adjustments and tissue repair activation. Macrophages also perform phagocytosis, in addition to the previously mentioned neutrophils and the next leukocyte recruited- dendritic cells.
Antigen Presentation
Once dendritic cells are recruited to the infection site, they consume additional debris through phagocytosis. Furthermore, they consume and intracellularly process antigens, which are key structures that provide pathogen identity and will be used for immune memory. After the dendritic cell consumes the antigen, the antigen is broken down into peptide fragments. The peptide fragments are attached to the major histocompatibility complex (MHC), which are glycoproteins on the dendritic cell’s membrane that hold the processed antigen (now called an epitope). The now combined MHC- epitope complex is brought to the surface of the dendritic cell, where it can then be presented to T cells. This antigen presentation is the switch from the innate to the adaptive immune system.
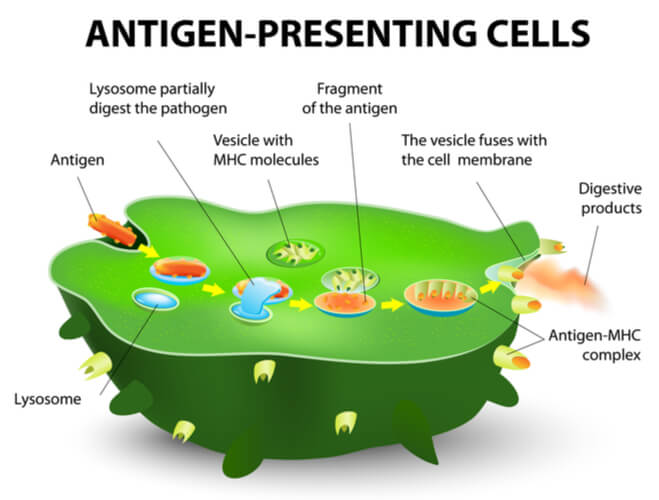
Before moving on to the adaptive immune system, it is important to note that there are variations in the MHC. The two major types in relation to T cells are class I and class II. Class I MHC binds to antigens from inside the endoplasmic reticulum while class II MHC binds to antigens from inside endosomes. The differentiation between class I and class II MHC will be important for T cell activation later on.
The Adaptive Immune System
Where the innate response is quick and non-specific, the adaptive response is slow, pathogen specific, and diverse. The adaptive response constitutes the recruitment of leukocytes that customize their response to foreign materials. This is where antibodies and the concept of memory are incorporated into the immune response.
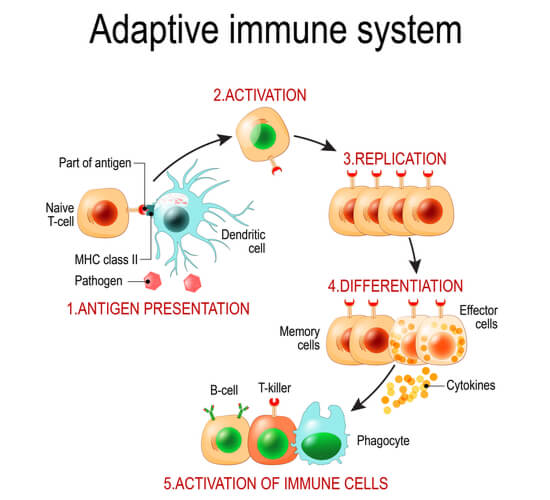
In the adaptive response, specific leukocytes known as lymphocytes are attracted to the area of interest. There are two types of lymphocytes: bone marrow- derived cells (B cell lymphocytes) and thymus- derived cells (T cell lymphocytes). Where all lymphocytes are produced in the bone marrow, B cells remain in the bone marrow through maturity while T cells mature in the thymus. (The thymus is found just under the sternum in humans.) When these lymphocytes need to the activated, they are moved to the spleen and lymph nodes. They then travel through the blood, lymph nodes, and additional immune organs to disperse throughout the body.
B cells are extremely important for antibody production. Antibodies are blood proteins that are created to attack specific pathogens. They belong to a group of immunoglobulins (Ig) and are divided into five classes (IgG, IgD, IgE, IgA, and IgM). B cells must first become activated by specific T cells (which will be discussed shortly) to become plasma cells. The now mature plasma cells are then responsible for creating and releasing antibodies in the pathogen’s presence.
T Cells
T cells make up between 60-70% of the lymphocytes in the blood. However, T cells were initially more difficult to isolate and define compared to B cells, as T cells do not bind directly to antigens like B cells. Instead, T cells must be presented with the antigen from antigen- presenting cells (such as the dendritic cells mentioned in the innate immune system) through antigen presentation. Antigen presentation is the link between the innate and adaptive immune responses. Once the MHC-peptide complex is on the surface of the dendritic cell, the T cell uses its T cell receptor to recognize the antigen and become activated. The types of MHC holding the antigen then determine which T cell type will be activated: CD8+ or CD4+.

(Note: There are a few additional T cell types in the body, such as regulatory T cells that suppress the immune response and prevent autoimmune diseases. However, this article will focus on the two major T cell types.)
CD8+ T Cells
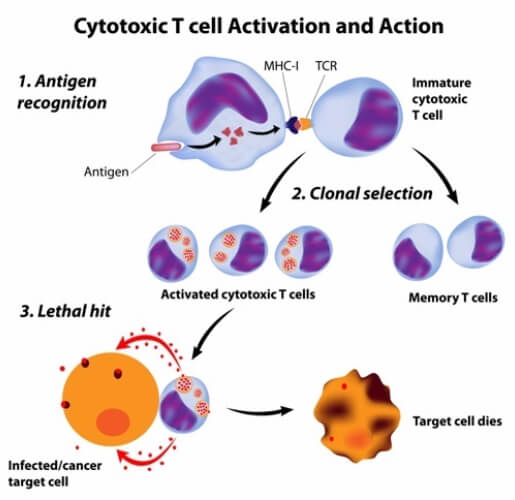
Class I MHC presentation activates CD8+ T cells. CD8+ T cells are classified as such because they contain the CD8 co-receptor on their membranes. About 40% of T cells have this receptor. When CD8+ T cells are activated, they multiply and differentiate into cytotoxic T cells (or killer T cells) through the process of clonal expansion. As the name may indicate, cytotoxic T cells are responsible for killing the host’s own cells that have been infected. Usually, this infection is caused by a virus or cancer.
Most nucleated cells in the human body already have class I MHC proteins on their surface. When an infection occurs in the cell, the cell moves part of the antigen to its MHC to alert circulating CD8+ T cells that it is infected. These activated CD8+ T cells (now cytotoxic cells) attach to the infected cell and secrete molecules that induce apoptosis (or programed cell death). This process is regarded as the “kiss of death” and is most common in host cells infected by viruses.
CD4+ T Cells
Class II MHC presentation activates CD4+ T cells. Just as with the CD8+ T cells, CD4+ T cells are named as such because they have the CD4 co-receptor on their membranes. About 50- 60% of T cells have this receptor. When CD4+ T cells are activated, they multiple and differentiate into helper T cells. Helper T cells are responsible for assisting the immune response by activating further immune cells when the body is attacked from extracellular pathogens, such as bacteria and fungi.
When extracellular pathogens enter the body, the innate immune response is first activated as previously described. Typically, the pathogens contain antigens that are directly processed onto class II MHC proteins. Following antigen presentation, the CD4+ T cells are activated into helper T cells. Helper T cells are further split into TH1 and TH2 cells. The dendritic cells determine which helper T cell it will become based on the toll like receptors activated initially. If they become TH1 cells, they assist in attacking the extracellular pathogens by increasing phagocytic activity of the macrophages already present at the site. Additionally, they recruit even more phagocytic cells, as well as activate additional cytotoxic T cells. Because TH1 cells increase cell- cell interactions, TH1 activation is part of the cell-mediated adaptive immune response.
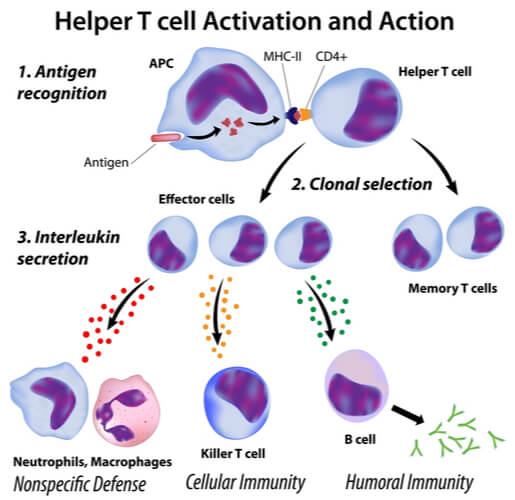
Meanwhile, TH2 cells aid B cell activation when extracellular pathogens are present in the body. This is part of the humoral adaptive immune response, as it leads to an increase in antibodies, which are released into the blood and lymph nodes. (Humoral is Latin for “fluid”.) By aiding B cell activation, the antibodies created attach to the pathogens. This attachment can lead to several outcomes. They can cause opsonization to the pathogen by coating it so that it will be readily eaten by phagocytes. Also, they can neutralize the pathogen by blocking it from infecting human cells. Additionally, they can cause agglutination to the pathogens by sticking them together (thus decreasing their ability to infect human cells). Lastly, they can co-stimulate complement proteins. Complement proteins are found in the bloodstream and attack pathogens by puncturing their membranes with holes.
T Cells in Hypersensitivity
Helper T cells also play a role in hypersensitivity reactions, which are the body’s attack on self-antigens, environmental antigens, and microbes that lead to over- damage in the body. There are four types of hypersensitivities: immediate, antibody- mediated, immune complex- mediated, and T cell- mediated (types I-IV, respectively). T cells can play numerous roles in these reactions.
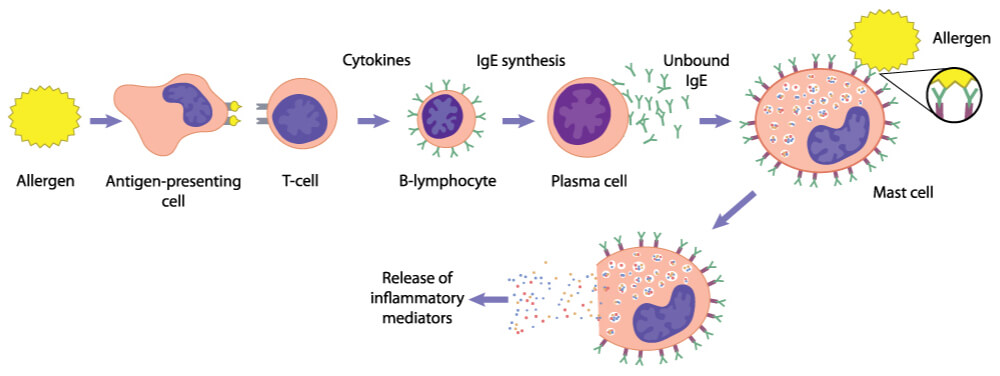
In immediate (type I) hypersensitivity, TH2 cells increase the production of IgE antibodies by activating B cells. The IgE antibodies bind to mast cells so that when the antigen attaches, the mast cells release mediators that result in allergy symptoms.
Meanwhile, in antibody- mediated (type II) hypersensitivity, antibodies bind to antigens fixed on tissues or cell surfaces. This increases tissue inflammation and phagocytosis of the host cells. Additionally, in immune complex- mediated (type III) hypersensitivity, the antibodies form complexes that increase inflammation (through complement activation) and cause further tissues injuries.
Lastly, in T cell mediated (type IV) hypersensitivity, TH1 and TH17 (another type of helper T cell) release cytokines to attract neutrophils and macrophages. This attraction can lead to tissue injury as CD8+ T cells are recruited to kill host cells.
T Cell Memory
The adaptive immune response is highly specialized in vertebrates, as it has memory. This is where activated B and T cells from the primary encounter produce daughter cells known as memory cells. These cells typically reside in the spleen and lymphatic system for months to years. When the antigen from the primary encounter returns to the body, the secondary immune response activates to quickly remove the infection. This secondary response is very strong and fast in comparison to the primary response.
Because of this memory, people can be immune to diseases that they have already had. Humans have taken advantage of this memory by creating vaccinations as a result. During the vaccination process, the body can be injected with an antigen from a weak or altered pathogen. This activates the immune system just enough to develop a response to the disease and create memory cells. This then allows the body to be ready for a fast secondary immune response if it were to be infected with the disease in the future. By creating this fast secondary immune response, the disease can be highly- to- entirely diminished, so that serious and life-threatening symptoms are less likely to develop.
While this concept has been revolutionary for the human race- as it resulted in the complete eradication of highly fatal diseases such as small pox– many vaccinations must be regularly updated. This includes the flu vaccination, as it is known to rapidly evolve.
Diseases Involving T Cells
As a major component of the immune system, diseases affecting T cells quickly become serious and often fatal. The most prominent of these diseases is HIV, with T cell lymphoma and T cell mediated rejection also posing as life threatening complications. Currently, these diseases do not have treatments. However, recent advancements in stem cell research and additional therapies have led to hopeful new avenues.
Human Immunodeficiency Virus (HIV)
The human immunodeficiency virus (HIV) is a spherical retrovirus surrounded by a liquid envelope that targets the immune and central nervous systems. It uses the CD4 molecule (in addition to additional receptors, such as CCR5 and CXCR4) on macrophages and CD4+ T cells as a path for cell entry. After HIV fuses with the cell membrane by connecting to these receptors, it injects its genome into the cell cytoplasm. The genome contains a gene known as nef, which activates intracellular kinase activity. This can result in affected T cell activation, viral replication, and viral infectivity. Additionally, it reduces the CD4+ and MHC molecules in the cells it infects.
Once inside the cell, the genome forms complementary DNA (cDNA) through reverse transcription. In dividing T cells, this cDNA is well integrated into the cell’s genome, where the now provirus can remain silent for months to years. The proviral DNA can also be transcribed to produce its own particles on the infected cell’s membrane that can lead to cell death.

Memory CD4+ T cells are the first to be infected during the acute phase of HIV, where high cell death rates can deplete the large reservoir of lymphocytes. In the chronic phase of HIV, the virus disperses and activates the body’s immune response. This recruits dendritic cells that eat the virus containing T cells. The dendritic cells then present the now processed antigen to healthy CD4+ T cells. From here, the infection rapidly increases, and the virus quickly replicates throughout the body. The infection then spreads into additional helper T cells, macrophages, and dendritic cells. This leads to both humoral and cell-mediated immune activation, where additional helper T cells attack infected T cells. This creates a cycle when T cells become diminished in the body. However, during this initial phase, the immune response is still able to generally protect itself from new diseases.
In the next phase however, HIV continuously replicates and destroys the immune cells it infects. This is the clinical latency period, where CD4+ T cell concentrations steadily drop in the blood. Over time, CD4+ cells are destroyed faster than they can be reproduced. Eventually, the body results in acquired immunodeficiency syndrome (AIDS), which is defined as the depletion of CD4+ T cells. The now weakened immune system is more likely to succumb when additional pathogens invade the body. Therefore, HIV and AIDs itself does not cause death. However, patients with HIV/AIDS lack the immune response needed to protect itself when it does eventually become sick.
Currently, there is no set cure for HIV. Instead, the best treatments are prevention and slowing the disease down as much as possible. Antiretroviral drugs have been fairly successful in keeping HIV patients heathy for as long as possible. However, recent stem cell research may find that replacing the white blood cells lost with HIV- resistant versions may lead to a complete cure.
T Cell Lymphoma
T cell lymphoma is a type of cancer in which T cells are targeted. It is initially caused by the infection of the human T cell leukemia virus type 1 (HTLV-1), a type of retrovirus. It can result in transverse myelitis, which is a disease that results in demyelination of the central nervous system. Typically, those presenting with T cell lymphoma have skin lesions, lymphadenopathy, hepatosplenomegaly, hypercalcemia, and various malignancies in the blood. The cells infected also have high concentrations of CD25. As an extremely aggressive tumor, patients typically last 8 months past diagnosis. Motivated to overcome this poor prognosis, multiple studies have been conducted to find viable treatments, including monoclonal antibodies, histone deacetylase inhibitors, anti-metabolites, and additional immunomodulary drugs.
T Cell Mediated Rejection
Transplanted organs can be rejected in the new body due to cell- mediated rejection and/or antibody- mediated rejection. This is usually when the host’s T cells do not recognize the new organ’s cells as its own, resulting in immune system activation. Cytotoxic T cells kill the new organ’s cells, which can lead to parenchymal and endothelial cell death. CD4+ T cells activate inflammation, allowing lymphocytes and macrophages to flood the new organ. Additionally, microphages can attack the cell and blood vessels, leading to diminished oxygen supply in the organ.
In order to protect the new organ, transplant recipients have to take immunosuppressive drugs to prevent their immune systems from activating. Typically, patients take these drugs for as long as they have the transplant. However, recent research may find that mixing the white blood cells from the compatible organ donor and the patient decreases organ reject. In this study, many of the patients were able to stop immunosuppressive drug treatments without organ rejection for at least two years.
Quiz
