Oxidizing Agent Definition
An oxidizing agent is a chemical substance which causes another chemical species to lose electrons. Oxidation means the loss of electrons, the loss of a hydrogen atom, or the addition of an oxygen atom. The oxidizing agent has the ability to accept or transfer those electrons.
Oxidizing Agent Overview
An oxidizing agent can be compared to a reducing agent, or a chemical which causes another molecule to gain electrons. The agent capable of oxidizing another species causes it to lose electrons. Alternatively, the oxidizing agent can be the addition of oxygen to a chemical species. Oxygen pulls electrons away from other parts of the molecule, effectively oxidizing the entire molecule. In other cases, as we will see in the examples, the oxidizing agent is separate from the reducing agent but allows the transfer of electrons to complete the Reduction-Oxidation reaction, or Redox reaction for short.
Redox reactions always consist of two half-reactions, whether or not they occur together. The reduction reaction happens when a chemical species gains electrons. These electrons must come from somewhere and are lost from another chemical species in a previous process. This process is known as oxidation. The oxidizer, or oxidizing agent, is responsible for removing these electrons. The agent can be directly involved in the reaction, or it can be a catalyst which simply drives the removal of electrons from a substance.
List of Oxidizing Agents
An oxidizing agent can be any chemical species which is prone to accepting electrons. Therefore, things like acids are usually oxidizing agents because of their propensity to take on more electrons. Below are several common oxidizing agents:
- Oxygen
- Fluorine
- Chlorine
- Nitric Acid
- Hydrogen Peroxide
- AND MANY MORE…
Oxidizing Agent Examples
Forming Salt in the Lab
Table salt is an extremely simple combination of two elements: sodium and chlorine. While most of the salt produced commercially is done through extracting premade salt from nature, it can be made in the laboratory. By combining solid metal sodium in an atmosphere of chlorine gas, the sodium will become oxidized. This oxidation reaction is coupled with a reduction reaction of the chlorine. In other words, sodium loses an electron, becoming the sodium cation (positive ion). The chlorine gains the electron, becoming a negative anion. Together, these two ions form the ionic compound of sodium chloride, or table salt. Interestingly, while table salt is mostly harmless, chlorine gas is an extremely toxic compound.
Part of the reason chlorine gas is so deadly is that it is an extremely powerful oxidizing agent. Chlorine is highly reactive and typically tries to draw away electrons. Although oxidation can turn metal into salt, it can also react dangerously with the numerous chemical reaction of the body, siphoning off much-needed electrons and causing chaos. Luckily, oxidizing agents only work in one direction. You don’t have to worry about being poisoned by your table salt.
The Fruit Battery
Another interesting oxidizing agent comes in the form of a classic classroom demonstration. The fruit battery, also known as the lemon or potato battery, is a form of electrical current produced the by effects of redox reactions. Two probes are placed on either side of a lemon or other fruit or vegetable. One probe, made from zinc, is connected through a light fixture to the other probe made of copper.
The zinc probe, in the presence of the acidity of the fruit, starts to dissolve into the fruit. It does so by being oxidized by the acids in the fruit. The acid acts as a catalyst, which allows some zinc atoms to shed their bonds with the other zinc by leaving behind the electrons which hold them in the matrix. The electrons, which are now building up in the zinc probe, try to distribute evenly along the probe. Meanwhile, on the copper probe, the copper is acting as a catalyst in reducing hydrogen ions into hydrogen gas. The copper deposits excess electrons onto hydrogen ions, which can then form covalent bonds with each other. This creates small bubbles around the copper probe.
Thus, on one side of the fruit battery, there is a demand for electrons and on the other side, there is an excess of electrons. The copper wire connecting the two probes through a light acts as a conductor, allowing an easy path for electrons to flow. As the electrons flow from the zinc to the copper, they can release some of their energy in the light bulb, and create light. The concepts described above can be seen in the image below, which is a diagram of any simple battery. The fruit battery, while some wrongly claim derives its power from the living fruit, functions like all batteries.
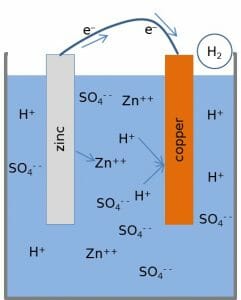
In this case, the oxidizing agent is not the direct recipient of the electrons but simply causes them to be removed from the zinc and passed through the wire. The reducing agent, which is the opposite of the oxidizing agent, is the copper wire because it catalyzes the transfer of the electrons into hydrogen molecules.
Oxidative Phosphorylation
One of the most important biochemical processes for all living animals is oxidative phosphorylation, or the transfer of electrons from nutrients to the molecules which provide energy for cells. Typically, the entire breakdown of food is a series of redox reactions, which have many different oxidizing agents and electron recipients. Oxidative phosphorylation is the last step of the process and occurs in the mitochondria of all plants and animals.
During oxidative phosphorylation, a series of proteins embedded in the mitochondrial membrane catalyze oxidation reactions and channel the electrons to other proteins. These proteins catalyze reduction reactions of ATP and other energy-providing molecules. This complex series of redox reactions use many proteins but operate in much the same way that the battery does. However, instead of releasing energy in the form of light, the energy is mostly trapped in the formation of new bonds. Some of the energy is released as heat, which is why the mitochondria are considerably hotter than the rest of the cell.
Quiz
