Ionic bonds are one of the two main types of chemical bonds. They form as a result of electrostatic attraction between oppositely charged ions and usually occur between metals and non-metals. When lots of ions bind together, they form a giant, regular, 3D structure called the ionic lattice, or crystal lattice.
What is an Ionic Bond?
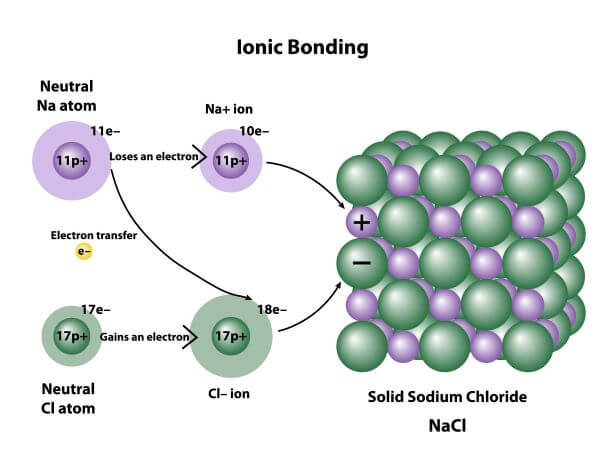
An ionic bond is a type of chemical bond formed by electrostatic attraction between two oppositely-charged ions. These ions are created by the transfer of valence electrons between two atoms, usually a metal and a non-metal.
How Are Ions Created?
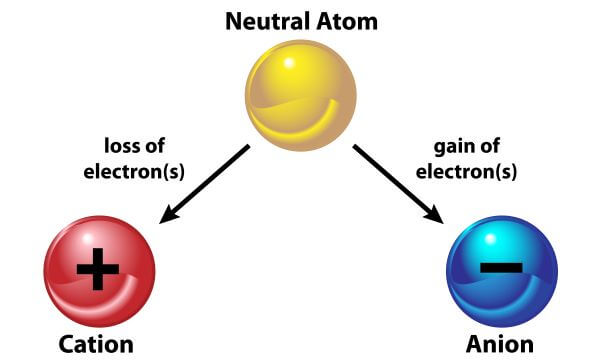
Ions are created when an atom loses or gains an electron. An atom that gains an electron becomes negatively charged, and is called an anion. An atom that loses an electron becomes a positively-charged cation.
During ionic bonding, two atoms (usually a metal and a non-metal) exchange valence electrons. One atom acts as an electron donor, and the other as an electron acceptor. This process is called electron transfer and creates two oppositely-charged ions.
How Are Ionic Bonds Formed?
Oppositely-charged ions have a strong electrostatic attraction between them. This attraction is the ionic bond, and it allows a positive ion and a negative ion to form a stable ionic compound with a neutral charge.
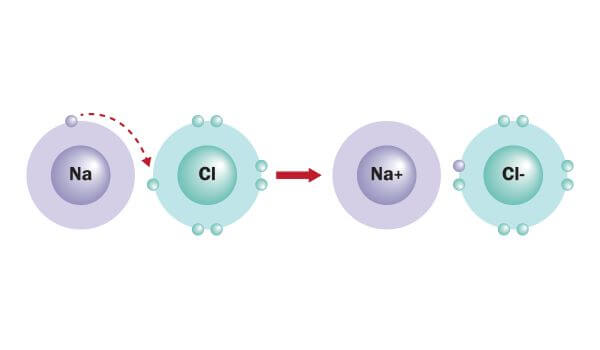
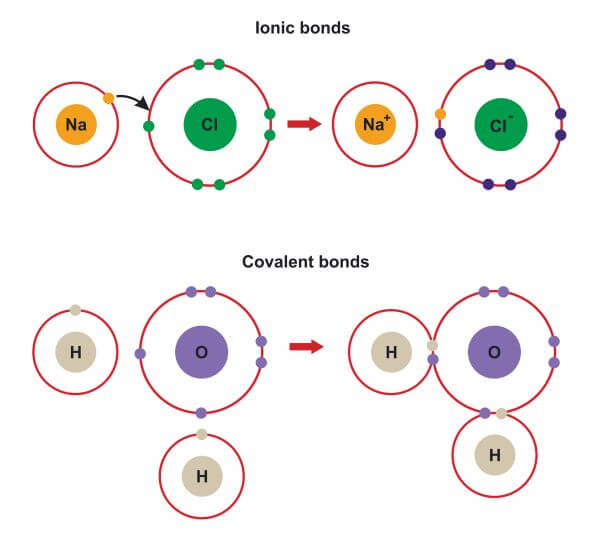
For example, when a sodium atom meets a chlorine atom, the sodium donates one valence electron to the chlorine. This creates a positively-charged sodium ion and a negatively-charged chlorine ion. The electrostatic attraction between them forms an ionic bond, resulting in a stable ionic compound called sodium chloride (AKA table salt).
Examples of Ionic Bonds
Some ionic bond examples include:
- NaCl: sodium chloride
- NaBr: sodium bromide
- NaF: sodium fluoride
- NaI: sodium iodide
- KF: potassium fluoride
- KCl: potassium chloride
- KI: potassium iodide
- KBr: potassium bromide
- LiI: lithium iodide
- Li2O: lithium oxide
- MgO: magnesium oxide
- MgS: magnesium sulfide
- MgSe: magnesium selenide
- CaCl: calcium chloride
- CaO: Calcium oxide
- CaSe: Calcium selenide
What is an Ionic Compound?
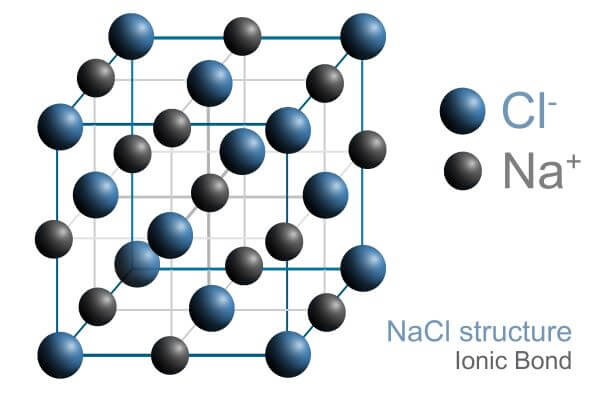
An ionic compound usually consists of a metal and a non-metal. When lots of ions come together, they form large structures called ionic lattices. Ions in an ionic lattice arrange themselves in a regular, 3D shape with oppositely charged ions next to one another. This structure is also sometimes referred to as a crystal lattice.
Properties of Ionic Compounds
In an ionic lattice, the strong electrostatic attraction between the oppositely charged ions acts in all directions, giving them a unique set of properties.
Ionic Compounds Have High Melting and Boiling Points
Ionic compounds have high melting and boiling points. This is because it takes a lot of energy to break the ionic bonds, thanks to the strong electrostatic attraction between oppositely charged ions.

Ionic Compounds Shatter Easily
Ionic compounds are hard but brittle. It takes a lot of force to break the ionic bonds that hold them together but, if enough force is applied, they shatter easily. This happens because breaking the ionic bonds brings ions of the same charge together. The strong repellent forces that exist between ions of the same charge makes them fly apart, causing the ionic compound to shatter.
Ionic Compounds Conduct Electricity
A substance can conduct electricity if it contains charged particles that are free to move about. All ionic compounds contain charged particles (ions), but they cannot conduct electricity in their solid form because the ions are not able to move. An ionic substance can only conduct electricity if it has melted or been dissolved in water, allowing the ions to move around.
What’s the Difference Between an Ionic Bond and a Covalent Bond?
The two main types of chemical bonds are ionic bonds and covalent bonds, but there are some key differences between the two.
| Ionic bonds | Covalent bonds |
|---|---|
| Bond between metals and non-metals | Bond between non-metals |
| Involves complete transfer of electrons | Involves sharing of electrons |
| Occurs between ions with considerably different electronegativities | Occurs between atoms with similar electronegativities |
Whereas ionic bonds involve the complete transfer of electrons between atoms, covalent bonds are formed when two atoms share electrons. This usually takes place between atoms of the same element, or between two elements that are close to one another in the periodic table. Covalent bonds are most likely to form between two atoms with similar electronegativities (i.e. those with a similar ability to attract electrons). They usually occur between two non-metals, though they may also be observed between metals and non-metals.
Ionic bonds are generally stronger than covalent bonds because of the electrostatic attraction that exists between oppositely charged ions.
