Facilitated Diffusion Definition
Facilitated diffusion is a form of facilitated transport involving the passive movement of molecules along their concentration gradient, guided by the presence of another molecule – usually an integral membrane protein forming a pore or channel.
Facilitated diffusion does not directly involve high-energy molecules like adenosine triphosphate (ATP) or guanosine triphosphate (GTP) since the molecules are moving along their concentration gradient.
Factors Affecting Diffusion
The driving force behind diffusion of fluids is simply the probability behind Brownian motion. All molecules have some degree of erratic, random movement, largely dependent on temperature. As temperature increases, the energy of these molecules increases.
When a substance is highly concentrated in a certain region, molecular movement, especially at the periphery, will lead to the gradual spread of the substance. When all the molecules within the region are moving randomly, some are bound to move outwards, into a region where its concentration is low. On the other hand, it is less likely that random molecular movement will result in the directional movement from a region of low concentration specifically towards regions of high concentration.
For instance, when someone walks into a room wearing a strong perfume, the odorous molecules diffuse outwards, from the skin or clothes. The people in the room perceive some of these randomly moving molecules when they trigger the sensory receptors in the nose. When there is a high density of scented molecules in a region, there is a chance that a few will move away due to the innate kinetic energy of these molecules. However, the likelihood that these few stray molecules will move in a directed manner, back towards the sleeve or cuff of the person wearing the perfume is relatively small. The end result is a cloud of progressively decreasing concentration away from the person wearing the perfume.
As seen in the example, the diffusion of a molecule needs a concentration gradient. If everyone in the room wears the same perfume, there would be a minimal effect from a new person entering the room. In addition, temperature increases the rate of diffusion. So, on hot days the perfume would diffuse quickly across the room. Diffusion is also dependent on the size of the molecule itself and the nature of the medium.
However, it does not depend on the concentration of any other substance in the medium. In the previous example, the aftershave of the person next to you will not influence the rate of diffusion of the perfume towards you. Though this could be an unpleasant experience, independent diffusion is an important property of molecules that allows cells to take in nutrients (diffusing in one direction), while at the same time, expelling metabolic waste products (diffusing outwards in the opposite direction).
Facilitated Diffusion Across Membranes
Diffusion is ubiquitous across the biosphere. It is seen in the movement of air and water, and is a necessary force driving global weather patterns. Within living systems, the presence of lipid-based membranes creates compartments that allow the selective concentration of water-soluble substances. For instance, mitochondrial membranes can create 2 distinct regions within the organelle – the inner matrix and the inter-membrane space. Each of these sub-compartments has a specific composition and function, distinct from the adjoining spaces. The generation of order in this manner is one of the hallmarks of nearly every unit of the living world – from organelles within a cell to entire organ systems and organisms.
However, this automatically means that ions, small molecules, proteins and other solutes have differential concentrations across lipid bilayers. Moreover, polar, charged or hydrophilic molecules cannot traverse biological membranes. While this is useful for maintaining the integrity of each compartment, it is equally necessary for molecules to move across membranes, along their concentration gradient, when needed.
Diffusion of Gases
An excellent example of this is the movement of oxygen and carbon dioxide in actively respiring tissues and cells. These cells need the input of oxygen and glucose while carbon dioxide needs to be removed and expelled from the body. Since each of these molecules are moving from regions of high concentration towards areas with low concentration, there is no direct involvement of ATP or other energy currency molecules. However, they do need to cross multiple lipid bilayers – from mitochondrial membranes, to the plasma membrane of the cell, and then the lipid bilayers of endothelial cells lining blood capillaries, the plasma membranes of red blood cells and finally the membranes of cells forming the alveolar sacs in lungs.
Need for Facilitated Diffusion
Cell membranes are only freely permeable to a very limited class of molecules. They must be small in size, and non-polar. While this allows molecules like water, oxygen and carbon dioxide to diffuse across membranes, it precludes practically every biopolymer, most nutrients and many important small molecules.
For instance, glucose is a relatively large molecule that cannot diffuse directly through the lipid bilayer. Similarly, important ions like sodium, potassium or calcium ions are charged and therefore repelled by the lipophilic core of cell membranes. Amino acids and nucleic acids are polar, often charged and too large to use simple diffusion to enter and exit cells. Occasionally, even the bulk movement of water across membranes cannot occur quickly through the lipid bilayer.
In these situations, facilitated diffusion, through integral membrane proteins, becomes important. These transmembrane proteins are usually of two types – those that act like carriers and those that form channels across the membrane.
Carriers and Channels
The study of integral membrane proteins is always difficult, since they are made of long hydrophobic stretches interspersed with hydrophilic regions. Crystallizing these proteins in order to understand their structure is fraught with difficulty. However, many of these proteins have been characterized through ingenious methods and we have some understanding of their activity.
Carrier proteins involved in facilitated diffusion often have two conformations. The binding of a molecule on one side of the membrane induces a change in the three-dimensional structure of the protein, which allows the passage of the molecule through to the other side.
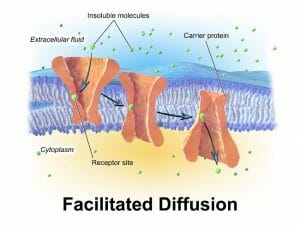
The image shows how a specific molecule (represented as a green ovoid particle) can induce binding-related conformational change in the carrier protein, creating a passage into the cell.
Proteins that form channels, on the other hand, have minute pores that selectively allow certain molecules to pass through. There are a number of mechanisms that determine the fit between a molecule and its channel proteins – from size, to charge and the ability to interact with the amino acid side chains lining the pore. Some channel proteins can show a thousand-fold preference for one molecule over other biochemically similar substances.
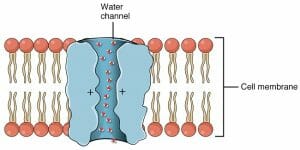
The image is a representation of an aquaporin molecule – protein channels that allow the quick bulk movement of water.
Examples of Facilitated Diffusion
A number of important molecules undergo facilitated diffusion to move between cells and subcellular organelles.
Glucose Transporter
When food is digested, there is a high concentration of glucose within the small intestine. This is transported through the membranes of the cells of the alimentary canal, towards the endothelial cells lining blood capillaries. Thereafter, glucose is transported throughout the body by the circulatory system. When blood flows through tissues that need energy, glucose traverses the endothelial cell membranes again and enters cells with low glucose concentration. Occasionally, when blood sugar levels drop, the movement can occur in reverse – from body tissues into blood circulation. For instance, hepatic cells can generate glucose even from non-carbohydrate sources to maintain a basal blood sugar concentration and prevent hypoglycemia.
The glucose transporter that facilitates this movement is a carrier protein that has two major conformational structures. While the exact three-dimensional structure is not known, the binding of glucose probably causes a conformational change that makes the binding site face the interior of the cell. When glucose is released into the cell, the transporter returns to its original conformation.
Ion Channels
Ion channels have been extensively studied in excitatory cells like neurons and muscle fibers since the movement of ions across the membrane is an integral part of their function. These channel proteins form pores on the lipid bilayer that can be either in the open or closed conformation, depending on the electrical potential of the cell and the binding of ligands. In this sense, these proteins are called ‘gated’ channels.
The presence of ion pumps in most cells ensures that the ionic composition of the extracellular fluid is different from the cytosol. The resting potential of any cell is driven by this process, with an excess of sodium ions in the extracellular region and an excess of potassium ions within the cell. The electrical and concentration gradient generated in this manner is used for the propagation of action potentials along neurons and the contractility of muscle cells.
When a small change in the voltage of a cell occurs, sodium ion channels open and allow the rapid ingress of sodium ions into the cell. This, in turn, induces the opening of potassium ion channels, allowing these ions to move outward, demonstrating that the diffusion of one substance can occur independently of another. In a few milliseconds, a region in the cell membrane can undergo large changes in voltage – from -75 mV to +30 mV.
The binding of neurotransmitters like acetylcholine to receptors on muscle cells changes the permeability of ligand-gated ion channels. The transmembrane channel is made of multiple subunits arranged like a closed cylinder. The binding of the ligand (acetylcholine) alters the conformation of the hydrophobic side chains that block the central passage. This leads to the rapid influx of sodium ions into the muscle cell. The change in the electric potential of the cell further results in the opening of calcium ion channels, which then lead to the contraction of the muscle fiber.
Aquaporins
Like other transmembrane proteins, aquaporins have not been fully characterized. However, it is known that there are many such channels for the rapid passage of water molecules in nearly every cell. These highly conserved proteins are present in bacteria, plants, fungi and animals. Mutations in the proteins forming aquaporins can lead to diseases like diabetes insipidus.
Related Biology Terms
- Brownian Motion – Random fluctuations in the velocity of particles in a fluid medium usually arising from intermolecular collisions.
- Hypoglycemia – Condition characterized by low blood glucose levels.
- Integral Membrane Protein – Proteins that are structurally and functionally an integral part of a biological membrane. Can traverse the entire width of the membrane or be attached through a small linker region.
- Partial Pressure – Hypothetical measure of the concentration of one gas in a mixture of gases.
Quiz
1. Which of these statements about facilitated diffusion of molecules is true?
A. Does not directly involve ATP
B. Needs the presence of another molecule
C. Necessary for the diffusion of polar molecules across a membrane
D. All of the above
2. Which of these factors affects the rate of diffusion?
A. Temperature
B. Viscosity of medium
C. Size of particles
D. All of the above
3. Which of these statements is NOT true?
A. Glucose undergoes facilitated diffusion through a transmembrane channel
B. Water can move across a membrane even in the absence of aquaporins
C. The potassium ion transporter has a thousand-fold greater affinity for potassium ions over sodium ions
D. All of the above
