Dehydration Synthesis Definition
Dehydration synthesis refers to the formation of larger molecules from smaller reactants, accompanied by the loss of a water molecule. Many reactions involving dehydration synthesis are associated with the formation of biological polymers where the addition of each monomer is accompanied by the elimination of one molecule of water.
Dehydration reactions are a subset of condensation reactions where two functional groups combine to form a covalent bond along with the release of a small molecule such as water, HCl, methanol or acetic acid. Though all these small molecules are frequently seen in large-scale industrial synthesis of organic molecules, in biological systems, water is the most frequent byproduct of a condensation reaction.
One common dehydration reaction involving a simple molecule is the formation of symmetric ethers from alcohol condensation. This is a reaction catalyzed by the presence of an acid and therefore occurs at a pH < 7. Given below is the formation of ethoxyethane from ethanol through dehydration synthesis.
2 C2H5OH ↔ C4H10O + H2O
The double-sided arrow indicates that this is a reversible reaction and can proceed in both directions. The reaction mixture reaches equilibrium between the reactants and products.
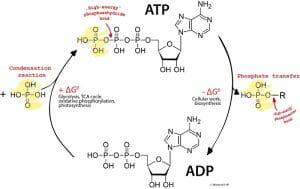
The other important and ubiquitous reaction is the addition of high-energy phosphate bonds to nucleosides such as adenosine or guanosine to give rise to adenosine triphosphate (ATP) and guanosine triphosphate (GTP).
The image shows the formation of a high-energy phosphate bond in ATP through a condensation reaction between adenosine diphosphate and a phosphate group as well as the reverse reaction involving the hydrolysis of that bond.
Types of Dehydration Synthesis
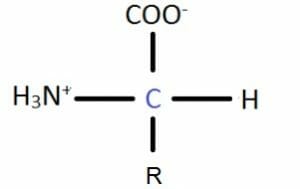
Dehydration synthesis can be classified based on a number of criteria. They can be grouped based on the nature of the reactants. Some reactants are molecules that have two functional groups, which can react with one another. For instance, amino acids contain an amine group and a carboxylic acid functional group attached to the same carbon atom.
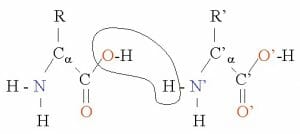
The amine group of one amino acid can react with the acid group of another to form an amide bond and release one molecule of water. The newly formed amino acid dimer again contains one free amine group and one free carboxylic acid group allowing the reaction to proceed with more amino acids.
Such bi-functional monomers, therefore, give rise to linear products with the monomers attached to each other end-to-end. Alternatively, the reactants could have multiple functional groups, which can create branched products, such as the formation of glycogen from glucose molecules.
Secondly, dehydration reactions can be classified on the nature of the catalyst. In the example on the formation of symmetrical ethers, the catalyst is a hydrogen ion. But for many reactions, especially within a living organism, the pH, salt concentrations and temperature cannot be altered. In these conditions, the presence of some other catalyst is important for driving a reversible reaction in one direction. Biological catalysts are called enzymes and often derive their name from the nature of the reaction they catalyze. For instance, enzymes that catalyze the formation of DNA from deoxyribonucleotides through condensation reactions are called DNA polymerases. Proteins are modified with carbohydrate moieties through glycosylases. Occasionally enzymes that catalyze a dehydration reaction are also named based on the nature of the enzyme itself. Ribosomes catalyze the formation of the amide bond (also known as the peptide bond) between two amino acids. Since the catalytic region within the ribosome is made predominantly of RNA rather than protein, it is also known as an RNA enzyme or ribozyme.
Alternatively, dehydration reactions can be classified based on the product they produce. In biological systems, most dehydration reactions create polymers. Therefore, these reactions can be grouped based on whether they create complex carbohydrates from simpler monosaccharides, form fatty acids from acetyl coA or synthesize proteins from amino acids.
Finally, dehydration reactions are also involved in the modification of biological molecules such as nucleosides, proteins and carbohydrates. These modifications include phosphorylation and glycosylation and are important for regulating the properties and functions of biopolymers. This is particularly crucial in many signaling cascades where protein kinases (enzymes that catalyze the phosphorylation of proteins) are involved.
Examples of Dehydration Synthesis
Dehydration reactions are involved in the industrial production of many substances that are used in daily life, such as polyesters. Polyester fibers are used to weave fabrics, yarns and ropes in addition to having a number of other uses including the making of bottles and insulating tapes. One common polyester is polyethylene terephthalate (PET) and gives its name to a class of recyclable bottles. PET is formed by dehydration synthesis from two monomers – ethylene glycol and teraphthalic acid.
In biological systems, dehydration synthesis reactions occur in every cell, especially since it is important for the formation of ATP. Nearly all biopolymers are also derived from this reaction.
Formation of Glycosidic Bonds
Glycosidic bonds are covalent bonds formed between a carbohydrate and any other molecule. Many of these involve a dehydration reaction. When maltose is formed from glucose, there is a glycosidic bond between two glucose molecules with the release of one molecule of water. Long polymers of glucose can be formed in a similar manner through a series of dehydration reactions to give rise to starch, cellulose or glycogen based on the position of the glycosidic bonds. Other disaccharides like sucrose and lactose are also formed through dehydration reactions between two monosaccharides.
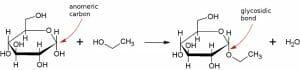
Additionally, glycosidic bonds are also involved when a carbohydrate is modified.
Here, a glucose molecule is reacting with methanol to give rise to ethyl glucoside.
Triglyceride Formation
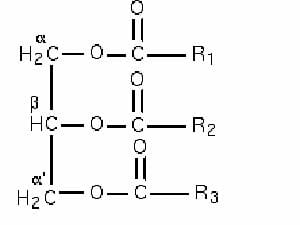
One of the intermediates of carbohydrate and fat metabolism is acetyl coA, a molecule where a two-carbon acetyl group is attached to coenzyme A. Though it is primarily intended to be a part of the Kreb’s cycle in the mitochondria, it can also be used to generate long chain fatty acids. These fatty acids then form triglycerides, which are an important energy storage molecule. Triglycerides derive their name from the fact that all three hydroxyl groups on glycerol undergo esterification with fatty acids. Each of the three fatty acids undergoes a dehydration reaction with the alcohol moieties on glycerol to generate one molecule of triglyceride.
One of the main reasons why triglycerides are considered a better storage medium than carbohydrates is their high energy density. They have a larger proportion of carbon atoms that can undergo oxidation and contain fewer oxygen molecules because fatty acids are generated from hydrocarbons. The removal of three water molecules in the process of forming a triglyceride further increases the energy density of the molecule.
In this image, R1, R2 and R3 refer to long chain hydrocarbons, each of which is attached to a carboxylic acid functional group. They form ester linkages with the hydroxyl groups attached to the α, β, and α’ of glycerol to give rise to a triglyceride.
Hydrolysis
Hydrolysis is the reverse of a dehydration reaction because it involves the breaking of a covalent bond through the addition of a molecule of water. Hydrolysis is catalyzed by a large group of enzymes called hydrolases. Among the most commonly known hydrolases are digestive enzymes. Digestion begins from the mouth, where salivary amylase breaks down starch molecules. This is why extended chewing of starchy foods gives rise to a sweet taste in the mouth. The action of salivary amylase generates monosaccharides. This is followed by the action of proteases in the stomach that begin the process of breaking peptide bonds in proteins. Digestion is continued by hydrolytic enzymes from the pancreas and small intestine acting on lipids, carbohydrates, nucleic acids and proteins. Each of these hydrolases has a specific name depending on the nature of its substrate. For instance, lipases act on lipids and nucleases on nucleic acids. A protease that severs peptide bonds from one end of the protein is called an exopeptidase and those that act on internal bonds are called endopeptidases. Similar enzymes are also present for intracellular digestion within lysosomes.
Additionally, there are specific enzymes that can reverse the post-translational modifications of proteins, such as phosphatases. These enzymes remove the phosphate group attached to a protein through a hydrolysis reaction. Similarly, ATPase enzymes catalyze the hydrolysis of the terminal phosphodiester bond in ATP, and are important for releasing the energy stored in the molecule. Many enzymes involved in hydrolysis contain a serine residue in their active site and are therefore known as serine hydrolases. These include most digestive enzymes and those involved in major metabolic pathways within the cell.
Related Biology Terms
- Aldol Condensation – A reaction in which a molecule containing a carbon-carbon double bond and an alcohol group reacts with a carbonyl compound through a dehydration reaction.
- Disaccharide – A sugar formed from two monosaccharides through a condensation reaction.
- Nucleophile – A reagent that is capable of donating a pair of electrons to an electrophile.
- Transesterification – A reaction where the organic group of an ester is exchanged with the group of another alcohol.
Quiz
1. Which of these involves the formation of an ester linkage through dehydration synthesis?
A. Creation of polyethylene terephthalate from ethylene glycol and teraphthalic acid
B. Creation of glycosylated carbohydrates
C. Creation of a peptide bond between two amino acids
D. All of the above
2. Which of these enzymes is involved in reversing the effects of a dehydration reaction?
A. Protein kinase that catalyzes the phosphorylation of a protein
B. DNA polymerase that catalyzes the formation of polynucleotide
C. Proteases that are involved in the digestion of proteins in the gastrointestinal tract
D. None of the above
3. Which of these properties contributes towards making triglycerides a good energy storage molecule?
A. High energy density
B. Greater proportion of carbon atoms in fatty acids when compared to carbohydrates
C. Esterification with glycerol
D. All of the above