Osmosis
In biology, osmosis refers to the movement of water. It is a passive process, meaning it requires no energy and happens automatically. The water moves from an area of greater concentration to an area of lesser concentration until equilibrium is reached. For cells, this movement is from one compartment to another through a semipermeable membrane. The solution is the area outside the cellular compartment, also called the extracellular environment.
Solute Concentration
Solute is the number of dissolved solids in a solution regardless of what they are. It can consist of proteins, carbohydrates, ions, hormones, etc. When discussing osmosis, comparison is made between the intracellular and extracellular (solution) solute concentrations. The concentration is also called the osmolarity of the solution. The difference between the number of dissolved solids creates an osmotic pressure gradient that forces water to move to achieve equilibrium between the inside and outside environments.
Hypotonic Solution
In a hypotonic solution, the solute concentration is lower than inside the cell. The prefix hypo means under or below in Latin. Under these conditions, the osmotic pressure gradient forces water into the cell. Depending on the amount of water that enters, the cell may look enlarged or bloated. If the water continues to move into the cell, it can stretch the cell membrane to the point the cell bursts (lyses) and dies.
Cells don’t have the ability to regulate their water content (remember that osmosis is a passive process), so they rely on the body to provide an environment where intracellular and extracellular solute concentrations are equal or isotonic. The body regulates the composition of extracellular fluid using the kidneys, adrenal glands, and the hypothalamus in the brain which triggers thirst and drives organisms to drink water. Slight fluctuations in the solute concentration of the extracellular fluid throughout the day cause small amounts of water to be exchanged between the intracellular and extracellular compartments to maintain homeostasis.
In contrast to hypotonic and isotonic solutions, a hypertonic solution has a higher solute concentration than inside the cell. When this happens, the osmotic gradient causes water to rush out of the cell and it becomes wrinkled or shriveled. If this happens to red blood cells, it is called crenation
Plant Cells
Plant cells respond the same way as animal cells in a hypotonic solution, but the affects may not be as severe. Plants have rigid cell walls made of cellulose covering the plasma membrane. This makes it difficult for the cell to lyse, but the increased pressure causes the sides of the cell to bulge out.
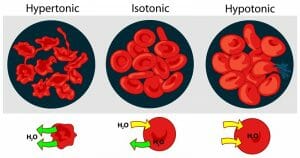
The image above shows the effects of osmotic pressure gradients on red blood cells.
References
- OpenStax College. (2018). Anatomy & Physiology. Houston, TX. OpenStax CNX. Retrieved from http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@8.119
- Tonicity. (n.d.). In Wikipedia. Retrieved April 25, 2018 from https://en.wikipedia.org/wiki/Tonicity
