Understanding Cell Pressure Gradients
In animals, cells are always striving to maintain an equilibrium between their internal (intracellular) environment and the surrounding (extracellular) environment. The barrier between the cell and the outside world is a semipermeable membrane called the cell membrane. Besides water, the extracellular environment for cells in the human body includes plasma, proteins, fats, glucose, waste products, ions, and other substances. These dissolved materials are called solutes. Similar solutes are also present inside cells.
Osmosis is a spontaneous homeostatic process where water moves from an area of low solute concentration to high solute concentration through a semipermeable membrane. This is a natural process reflecting the preference of systems to achieve and maintain equilibrium. The amount of water outside a cell compared to the inside creates an osmotic pressure gradient which causes water to move. In other words, if there are more solutes outside the cell than inside, water will move out of the cell to equalize the solute level inside. Conversely, more solutes inside the cell compared to the outside environment causes water to enter the cell. The process by which organisms maintain water balance is called osmoregulation.
Hypertonic Solutions
For a discussion about what happens to a cell in a hypertonic solution, ‘solution’ refers to the extracellular environment. Hyper is a Latin prefix meaning over or above. Therefore, a hypertonic solution has more solutes than the intracellular environment, so water will leave the cell to try to achieve equilibrium. If enough water is lost, the cell will take on a wrinkled or shriveled appearance. In red blood cells this is called crenation and the surface of the cells take on a scalloped appearance. A high amount of water loss can be damaging or even fatal for a cell.
How Some Organisms Overcome Hypertonic Solutions
Marine organisms often live in hypertonic environments compared to their internal body chemistry. Species can live in such environments because they have evolved adaptive mechanisms. Fish, for example, use the large surface area of their gills for gas exchange with the saltwater. However, due to osmosis, the cells in the gills continually lose water to the sea. The fish overcome this by drinking large amounts of saltwater and excreting the excess salt. This process allows them to maintain fluid homeostasis while living in a hypertonic environment.
Isotonic and Hypotonic Solutions
An isotonic solution has a solute concentration equal to that inside of the cell. This is a state of equilibrium and no water moves in or out through the semipermeable membrane. In contrast, a hypotonic solution has less solute than inside the cell, like putting a cell in distilled water. In this situation, water enters the cell, and if left uncontrolled it can cause the cell to burst (lyse) and die.
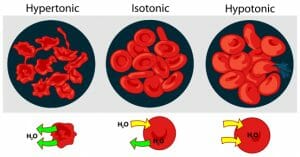
The image above shows what happens to red blood cells in hypertonic, isotonic, and hypotonic solutions. Note the movement of water based on the solute concentration of the extracellular fluid.
References
- OpenStax College. (2018). Anatomy & Physiology. Houston, TX. OpenStax CNX. Retrieved from http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@8.119
- Tonicity. (n.d.). In Wikipedia. Retrieved April 16, 2018 from https://en.wikipedia.org/wiki/Tonicity
