Thymine Definition
Thymine is one of the four nitrogenous nucleobases that form the basic building blocks of deoxyribonucleic acid (DNA).
Also known as 5-methyluracil, thymine (T) is a pyrimidine nucleobase, which pairs with adenine (A), a purine nucleobase. They are joined together as a base pair by two hydrogen bonds, which stabilize the nucleic acid structures in DNA. When stacked with the other base pair, guanine (G) and cytosine (C), the helical structure of DNA (or RNA) is formed.
In the structure of RNA, thymine is replaced by the uracil nucleobase. As suggested by its alternative name (5-methyluracil), thymine can be derived by methylation of uracil at the 5th carbon (a methyl branch –CH3 is added to the pyrimidine ring).
The combination of thymine, with the pentose sugar, deoxyribose, forms the nucleoside, deoxythymidine (alternatively named ’thymidine’). A nucleoside is a nucleotide without a phosphate group.
Mutation and Cancer
When exposed to ultraviolet radiation such as sunlight, covalent bonds are formed between adjacent thymine molecules on the same strand of DNA, creating thymine dimers. This process causes damage, by causing the DNA to form ‘kinks’. This inhibits the normal function of the DNA, which cannot then be replicated or transcribed.
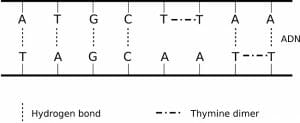
Fortunately, most cells are able to repair damaged DNA. This can be achieved in two ways: repair enzymes called photolyase can break the covalent bond, using light as an energy-source for bond cleavage. This process is called photoreactivation and is possible in most organisms, although not in placental mammals.
The second mechanism involves an excision enzyme, which removes the damaged section from a single strand of DNA. The excised nucleotides are then replaced by DNA polymerase and a final phosphodiester bond (the stabilizing structure of nucleic acids) is formed by DNA ligase.
Thymine Structure
The formula of thymine is C5H6N2O2.
Properties
It is a heterocyclic, aromatic, organic compound.
Heterocyclic compounds or ‘ring structures’ are cyclic compounds (the atoms in the compound are connected to form a ring), that have atoms of at least two different elements.
An ‘organic’ compound contains carbon, so a heterocyclic organic compound contains atoms of carbon and one or more additional elements such as sulphur, nitrogen or oxygen.
The term aromatic describes a molecule which is cyclic and planar (flat), with a ring of resonance bonds, which give the molecule added stability. This means that it does not break apart or react with other substances easily.
Atomic Structure
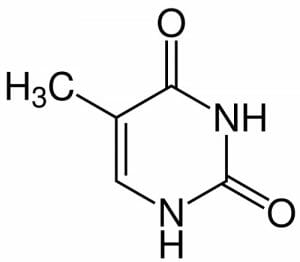
In the same way as the other pyrimidines, cytosine and uracil, thymine has nitrogen (N) at the 1’ and 3’; each one is bonded to hydrogen (H). The N molecule at the 3’ prime forms the glycosidic bond – the covalent bond which joins to the deoxyribose sugar to form the nucleoside, thymidine.
At the 2’ and 6’ positions are carbonyl groups and at the left hand side is a double bond.
Thymine has a methyl group at the 5’ position, distinguishing it from uracil, which has hydrogen at that position.
Related Biology Terms
- Adenine – The purine nucleobase that bonds with thymine in DNA and with uracil in RNA.
- Guanine – The purine nucelobase, which bonds with cytosine in DNA and RNA.
- Cytosine – The pyrimidine nucelobase, which bonds with guanine in DNA and RNA.
- DNA – The self-replicating, main component of chromosomes, which carries genetic information Deoxyribonucleic acid.
Quiz
1. Which nucleobase does thymine bond with in DNA strands?
A. Adenine
B. Cytosine
C. Guanine
2. What is the role of an excision enzyme?
A. To speed up the reproduction of DNA.
B. The begin the formation of the A-T bond.
C. To remove damaged strands of DNA.
D. To break the covalent bond formed between thymine dimers.
3. In the formation of the nucleoside, thymidine, what does the nitrogen atom at the 3’ position of thymine do?
A. Bonds to adenine
B. Bonds to deoxyribose
C. Bonds to ribose
D. Does not bond to anything
