Pyruvate Definition
Pyruvate is an important molecule that is present at the intersection of multiple biochemical pathways. It is commonly encountered as one of the end products of glycolysis, which is then transported to the mitochondria for participating the citric acid cycle. In the absence of oxygen, or when oxygen demand outstrips supply, pyruvate can undergo fermentation to produce lactate. Both pyruvate and lactate can be used to regenerate glucose as well. Pyruvate can also be involved in the anabolic synthesis of fatty acids and amino acids. There is also growing evidence that it can directly influence nuclear activity and epigenetic modifications, forming the interface between the genome and the metabolic state of the cell.
Pyruvate Structure
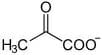
This molecule is the conjugate base of pyruvic acid, a three-carbon molecule containing a carboxylic acid group and a ketone functional group. The chemical formula for pyruvic acid is C3H4O3 and for its deprotonated form is C3H3O3. The carbon atom forming the carboxylic acid is often referred to as the first carbon atom, with the number increasing along the carbon backbone, away from the carboxylic acid terminus. In pyruvate, the ketone group is attached to the second carbon atom, also known as the α-carbon since it is closest to the main functional group; the third carbon comprises a methyl group.
It is, therefore, the simplest α-keto acid and according to official nomenclature by IUPAC, it is called α-keto propanoic acid. It contains three atoms that can act as hydrogen-bond donors and one atom that can be a hydrogen-bond acceptor. Like other keto acids, pyruvic acid can also tautomerize from its ketone form to its enol form, containing a double bond and an alcohol. This is particularly important in the last step of glycolysis.
Other α-keto acids involved in cellular respiration include oxaloacetic acid, α-keto glutaric acid and oxalosuccinic acid.
Generation of Pyruvate
Pyruvate is generated by two primary methods – through the glycolytic pathway, and through the metabolism of amino acids. While proteins supply nearly 10% of the body’s energy needs, only some amino acids are channeled through pyruvate into the cellular respiratory machinery. Those that do are classified as glucogenic amino acids, while others that generate acetyl-CoA or acetoacetate are classified as ketogenic amino acids. Lactate produced by anaerobic fermentation can also regenerate pyruvate, especially through the activity of enzymes in the liver. Other minor sources include the intermediates of the citric acid cycle.
Glycolysis
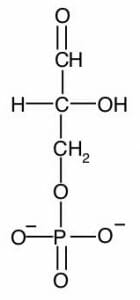
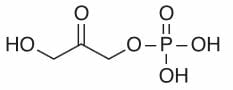
Glycolysis begins with the six-carbon monosaccharide – glucose. In the first few steps of this biochemical pathway, glucose undergoes phosphorylation and isomerization to produce fructose-6-phosphate. Another phosphorylation reaction facilitates the splitting of this hexose sugar into two 3-carbon molecules – glyceraldehyde phosphate (G3P) and dihydroxy acetone phosphate (DHAP). These initial steps require the input of energy and utilize two molecules of ATP for every molecule of glucose, but result in the major transformation of a hexose into two triose molecules.
Images show the chemical structures of G3P and DHAP. These isomers can be interconverted, especially through enzyme-mediated catalysis.
Thereafter, G3P is converted to pyruvic acid, which exists as its conjugate base at physiological concentration and pH. This process occurs through a set of five biochemical reactions, releasing two molecules of ATP and one molecule of NADH for each molecule of G3P.
The penultimate molecule in this chain of reactions is called phosphoenol pyruvate (PEP). PEP is the phosphorylated ester of the pyruvate in its enol isomeric form. PEP loses a phosphate group to generate pyruvate and the released phosphate moiety is transferred to ADP, forming ATP. This reaction is catalyzed by an enzyme called pyruvate kinase (PK). The reaction forms one of the rate-limiting steps of glycolysis that can determine the overall reaction rate, since it is one of the slower reactions in the chain. For all practical purposes, it is irreversible, unlike most enzyme-catalyzed reactions, especially since pyruvate is often quickly moved to the mitochondria or fermented to form lactate. When glucose needs to be generated from non-carbohydrate sources (gluconeogenesis), for instance, changing concentrations of reactants and products does not induce PK to catalyze the reverse reaction, forming PEP from pyruvate. In fact, during gluconeogenesis, PK is deactivated through phosphorylation and PEP is diverted towards a different cascade of reactions.
Amino Acid Metabolism
Six major amino acids can be metabolized to produce pyruvate – alanine, cysteine, serine, glycine, threonine and tryptophan. Of these, alanine and serine have three carbon atoms and are therefore the easiest to transform. These reactions involve a single enzyme that essentially catalyzes the replacement of the amine functional group with a ketone. The enzymes are also called transaminases for this reason. Cysteine, while also containing three carbon atoms, has to undergo an additional step to remove the sulfur atom.
Glycine, on the other hand, has only two carbon atoms. Therefore, it is first converted into a three-carbon amino acid – serine – before undergoing deamination. The action of serine dehydratase enzyme then catalyzes its conversion into pyruvate. In a similar strategy, three alkyl groups of tryptophan are first converted to alanine before being transformed into a pyruvate molecule through the action of alanine transaminase enzyme. Threonine follows an even longer path, first being converted to glycine, and then to serine before being acted on by serine dehydratase.
Functions of Pyruvate
The primary function of the molecule is to act as the transport molecule that carries carbon atoms into the mitochondria for complete oxidation to carbon dioxide. At the end of glycolysis in the cytoplasm, the pyruvate molecules generated from glucose are transported into the matrix of the mitochondria through two proteins: Mitochondrial Pyruvate Carriers 1 and 2 (MPC1, MPC2). Within the matrix of the mitochondria, an important multi-enzyme complex called pyruvate dehydrogenase complex (PDC) catalyzes decarboxylation and oxidation reactions in order to generate acetyl coenzyme A (acetyl-CoA).
The first enzyme in this complex is called pyruvate dehydrogenase and removes the carboxylic acid group (decarboxylates) the molecule. The result of this reaction leaves a two-carbon molecule containing a methyl group and a carbonyl group. The second and third enzymes of PDC then oxidize the carbonyl carbon and catalyze a covalent linkage to CoA through a thioester linkage. This thioester bond can be hydrolyzed with the release of energy. In fact, the hydrolysis of this bond releases more energy than the conversion of ATP to ADP and therefore also provides the initial impetus for the first steps of the citric acid cycle.
Recently, attention has been drawn to the role of pyruvate molecules in influencing genome-wide acetylation of histone molecules. Histone acetylation is an epigenetic modification that can change the overall transcriptional activity of the cell as well as influence the cell cycle and mitosis. This histone modification requires the presence of acetyl-CoA. Acetyl-coA is generated through PDC in the nucleus as well, through the transport of the entire enzyme complex from the mitochondria to the nucleus. The concentration of this complex in the nucleus is dependent on the cell cycle, the external environment, and the availability of growth factors and nutrients. In the absence of sufficient PDC activity, the transition of the cell towards mitosis (specifically, the synthesis of DNA during the S phase) is hampered.
Another enzyme associated with pyruvate metabolism that is also present in the nucleus is pyruvate kinase – the enzyme involved in the last reaction of glycolysis, generating pyruvate from PEP. This kinase plays an interesting role within the nucleus, phosphorylating nuclear proteins using PEP itself as a phosphate donor. This, in turn, leads to the generation of pyruvate, which can be used by the PDC to create acetyl-CoA. The phosphorylation of key residues in histones also enhances their acetylation, again a crucial step in the progression of the cell from the G1 to S phase of the cell cycle.
If aerobic respiration is not possible, pyruvate can be fermented to lactate in the cytoplasm to generate NADH and thereby increase the ATP availability for the cell. The enzyme involved in pyruvate fermentation can also catalyze the reverse reaction, forming pyruvate from lactate. This is particularly important in the cells of the liver where this is an essential process during the recovery period after exercise.
Additionally, pyruvate functions as one of the starting points for gluconeogenesis, allowing the cell to generate glucose from non-carbohydrate sources. This process is important for the functioning of the brain during fasting, since the tissues in the brain use glucose as the primary energy source. Pyruvate is also implicated in the generation of non-essential amino acids as well as in many biochemical pathways involving lipid metabolism.
Related Biology Terms
- Hexose – Monosaccharide that contains six carbon atoms, such as glucose or fructose.
- Kinase – An enzyme that catalyzes the transfer of a phosphate group (usually from ATP) to another molecule.
- Rate Limiting Reactions – The slowest steps in metabolic pathways that determine the rate of all the other reactions in the pathway.
- Tautomer – Isomers that exist together in equilibrium, with the migration of an atom or functional group within the molecule occurring frequently.
- Triose – Monosaccharide that contains three carbon atoms, such as glyceraldehyde.
Quiz
1. Which of these amino acids needs more than one enzyme to be converted to pyruvate?
A. Glycine
B. Alanine
C. Serine
D. All of the above
2. Why is the conversion of PEP to pyruvate one of the rate limiting steps in glycolysis?
A. It generates the most important molecule of glycolysis
B. It is one of the slower reactions in the pathway
C. The enzyme, pyruvate kinase, is present in low concentration
D. It is an irreversible reaction
3. Which of these is a function of pyruvate?
A. Fermentation to lactate during aerobic respiration
B. Epigenetic modifications of the mitochondrial genome
C. Participation in the generation of glucose
D. All of the above