[LS2-5] The Carbon Cycle, Photosynthesis and Respiration
This standard focuses on carbon as a central atom in the processes of photosynthesis and respiration, and how carbon molecules flow through the biosphere in a massive cycle.
Resources for this Standard:
Here’s the Actual Standard:
Develop a model to illustrate the role of photosynthesis and cellular respiration in the cycling of carbon among the biosphere, atmosphere, hydrosphere, and geosphere.
Standard Breakdown
This standard has 4 specific levels that we will look at in detail:
Biosphere
The biosphere includes everything on Earth, both living and non-living. Carbon cycles through both living and non-living parts of the biosphere. Starting in the atmosphere, carbon dioxide is used by plants to create glucose. Glucose travels through the food web, where it is used for energy. Some of the carbon is released as carbon dioxide back into the atmosphere. Other carbon remains bound in bonds – some of it becoming petroleum as it sinks into the pressurized environment of the Earth’s crust. Carbon also makes its way from the atmosphere and into the hydrosphere and geosphere – all of which are considered “carbon-sinks”, or places where carbon can be stored.
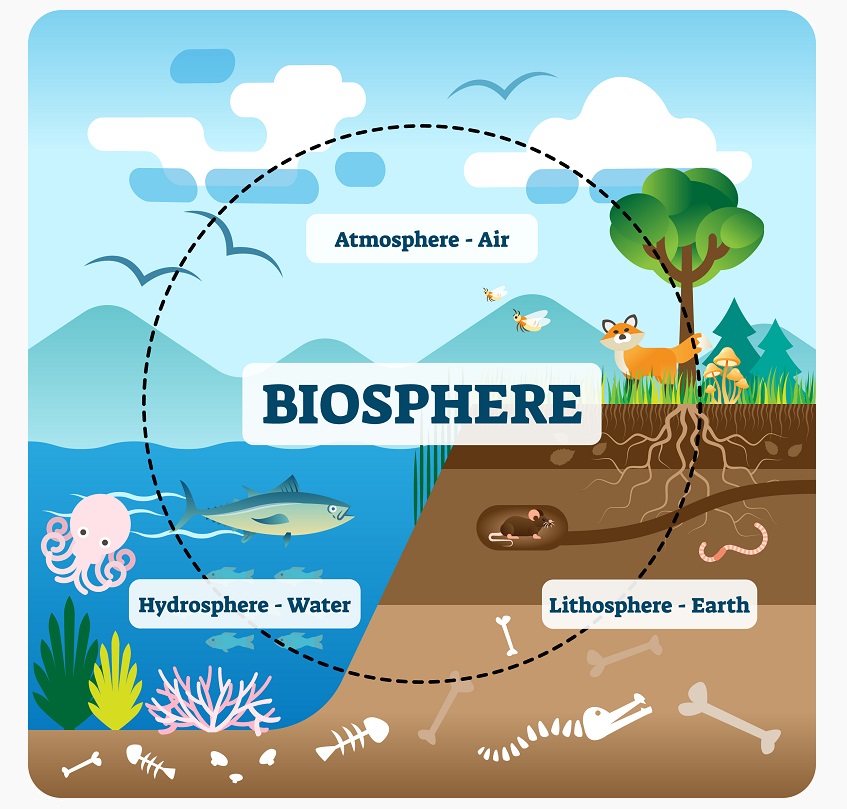
Atmosphere
The atmosphere is where a majority of the gaseous forms of carbon end up. Within the context of photosynthesis and respiration – this is mainly carbon dioxide. However, human activities also produce molecules like carbon monoxide and methane, both of which are also greenhouse gases. In the atmosphere, many carbon-based molecules serve as greenhouse gases, which reflect heat back onto the Earth. Too many greenhouse gases can drastically change how much heat is trapped, affecting global weather patterns and plant growth. However, the carbon in the atmosphere is also transferred to the hydrosphere and geosphere.

Hydrosphere
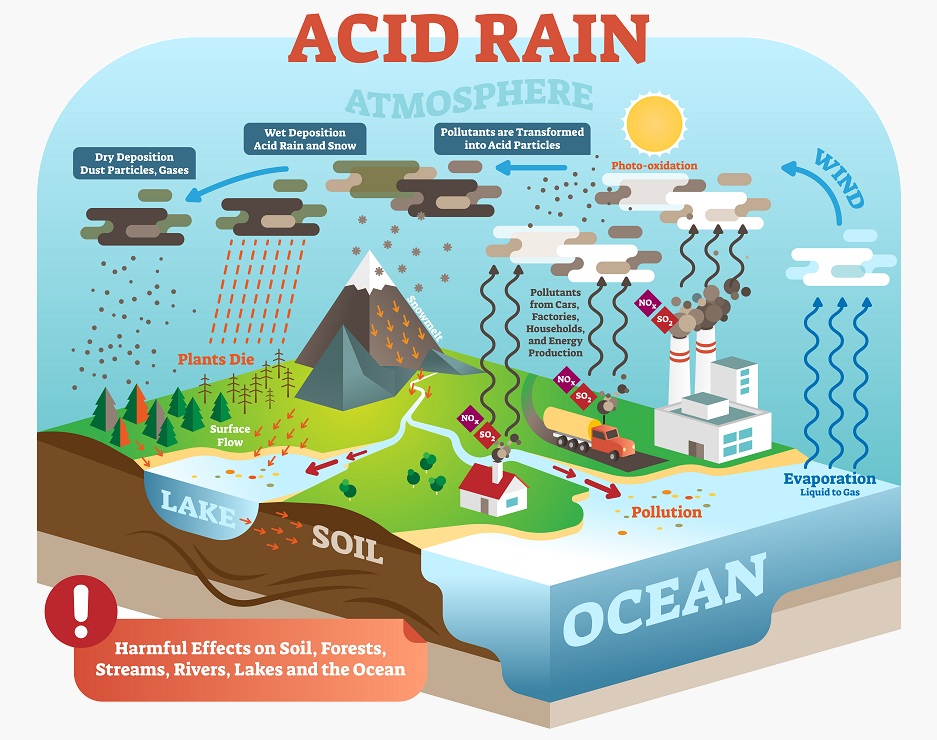
The hydrosphere is all of the interconnected water sources on the globe. Water is a universal solvent – because water is a polar molecule it can easily dissolve many other solutes. As such, water also allows a certain amount of carbon dioxide to be dissolved into water sources. Without humans, the oceans may have just acted as a reservoir that kept carbon dioxide at a more reasonable level. However, because of how much carbon dioxide and other greenhouse gases humans are creating, the oceans are now soaking up far too much carbon dioxide. In water, carbon dioxide dissolves and can form carbonic acid, which may be responsible for higher rates of coral and mollusk mortality in many areas. This is the same phenomenon that creates acid rain.
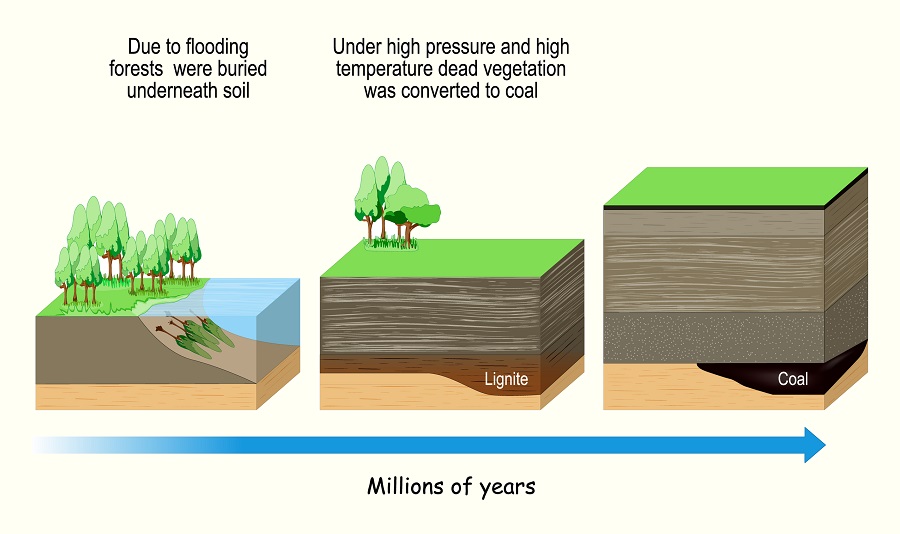
Geosphere
The geosphere includes inorganic carbon sinks that exist, such as the soil (lithosphere) and various types of porous rocks that can store carbon. One of the largest carbon reservoirs from the geosphere that we use is fossil fuels. Essentially, fossil fuels represent the carbon stored by plants and algae for hundreds of millions of years. These carbons, which were not released by respiration, make their way into the rock layers of the earth. With the right amounts of heat, compression, and time, the carbons joined together into longer molecules packed with energy. By burning these carbons, we are essentially releasing carbon that plants and algae stored millions of years ago.
A little clarification:
The standard contains this clarification statement:
Examples of models could include simulations and mathematical models.
Let’s look at this clarification a little closer:
Simulations and Mathematical Models
There are many video simulations online that can help explain this standard in a visual and auditory way. For more hands-on learning, you can do an experiment where you show the accumulation of carbon in water. This basic experiment works by using lime water (adding calcium hydroxide to water) as an indicator of carbon dioxide. As you breathe into the lime water, it becomes cloudy as the carbon dioxide reacts with the calcium hydroxide to precipitate calcium carbonate – chalk!

Good mathematical models involve measuring and analyzing the contributions of greenhouse gases to the atmosphere, as well as a carbon-for-carbon analysis of the processes of photosynthesis and respiration, which shows mathematically how the processes complement each other.
What to Avoid
This NGSS standard also contains the following Assessment Boundary:
Assessment does not include the specific chemical steps of photosynthesis and respiration.
Here’s a little more specificity on what that means:
Specific Chemical Steps:
For this standard, the actual processes of photosynthesis and respiration do not have to be memorized in detail. Instead, it is more important to show the movement and transformation of carbon as it weaves its way through the biological and inorganic world. While the chemical steps can help explain how carbon is changed and transformed, they should not be assessed for this standard.
