One method healthcare providers use to treat patients is via Intravenous (IV) therapy. Food, blood products, and medications are administered through a line (catheter) inserted into peripheral veins in the arms, hands, feet, or legs, or a large central vein. Peripherally inserted central catheters (PICC) are used when the IV access is needed over a longer period like for chemotherapy, long-term feeding, or extended antibiotic therapy.
IV solutions are another method to treat patients and are used to replace and control fluid and electrolyte levels in the body. These solutions are also called volume expanders. Patients suffer the loss of body fluid volume from excessive external or internal bleeding (hemorrhaging), severe burns, surgery, and dehydration, among other causes. Isotonic IV solutions restore fluid volume because they fill the tissues and maintain fluid volume more effectively than hypertonic or hypotonic solutions.
Body Fluids
Maintaining the proper balance of aqueous solutions is essential to life because that is where chemical reactions take place in the body. Body fluids are divided into three compartments: the intracellular fluid (ICF), extracellular fluid (ECF), and interstitial fluid (IF). The ECF comprises the IF and the fluid part of the blood called plasma.
In the body, water moves from one compartment to another by the process of osmosis which is dependent on the concentration of solute particles (ions, proteins, lipids, carbohydrates, etc.) in the ECF compared to the ICF. If the ECF has a greater solute concentration (hypertonic) than the ICF, water will leave the cell causing it to wrinkle or shrivel. When the ECF solute concentration is lower (hypotonic) than the ICF, water rushes into the cell making it swell and possibly explode (lyse). In isotonic solutions, the solute concentrations of the ICF and ECF are equal so there is only enough water movement in and out of the cell to maintain equilibrium.
Types of Isotonic IV Solutions
IV solutions consist of water and various amounts of dissolved ions such as sodium (Na+), chloride (Cl–), potassium (K+), magnesium (Mg2+), calcium (Ca2+), buffers, and other components. The solution the patient receives depends on their medical condition. The solutions shown below are all isotonic but vary slightly depending on the patient needs and appropriate uses.
Normal Saline (NS)
This IV fluid contains 0.9% NaCl in water. It is used to increase the volume of plasma when there is no hemorrhaging and there are enough red cells in the blood. Medical conditions treated with NS include fluid replacement for patients with diabetic ketoacidosis (DKA), treatment for too little sodium in the blood (hyponatremia), blood transfusions, metabolic alkalosis, and treatment for too much calcium in the blood (hypercalcemia). It is important to note that because NS replaces extracellular fluid, it isn’t used in patients with heart failure, too much sodium in their blood (hypernatremia), or fluid retention (edema).
Lactated Ringer’s (LR)
LR is used to replace fluids and as a pH buffer. It is particularly useful in cases of acute blood loss, lower gastrointestinal tract fluid loss, burns, surgery, and dehydration. It’s the same as NS but also has the electrolytes K+ and Ca2+ and a buffer called lactate (a salt of lactic acid). LR is not used in patients with renal failure because it may cause too much K+ to build up in the body (hyperkalemia) or those with liver disease because they can’t metabolize the lactate.
D5W
This IV solution has a two-fold effect. It is an isotonic solution when it is administered but becomes hypotonic as the patient’s body metabolizes the 5% dextrose it contains. In other words, D5W is isotonic in the bag and “physiologically hypotonic.” Because of the hypotonicity, this IV fluid raises the total body fluid volume and is used rehydrate patients, assist with excretory processes, and treat hypernatremia.
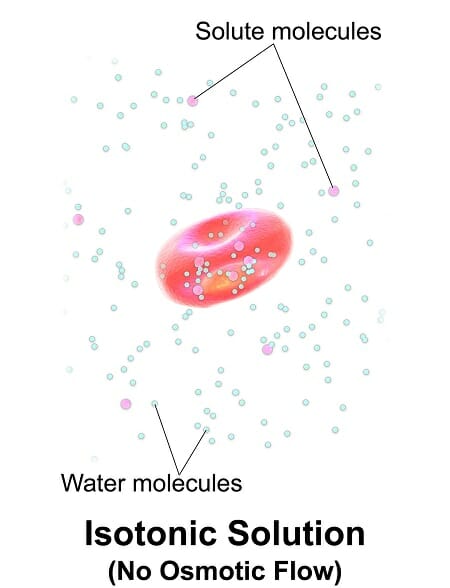
The image above shows a human red blood cell in an isotonic solution. When the extracellular solution has the same solute concentration as inside the cell, the solution is isotonic. The solute concentration of isotonic IV solutions is similar to the intracellular environment.
References
- Hoorn, E. J. (2017). Intravenous fluids: balancing solutions. Journal of Nephrology, 30(4), 485–492. https://doi.org/10.1007/s40620-016-0363-9
- Intravenous therapy. (2018, April 10). In Wikipedia. Retrieved from https://en.wikipedia.org/w/index.php?title=Intravenous_therapy&oldid=835778719
- Vera, M. (2012, February 8). Intravenous (IV) Fluids and Solutions Quick Reference Guide & Cheat Sheet. Retrieved May 7, 2018, from https://nurseslabs.com/iv-fluidsolution-quick-reference-guide-cheat-sheet/
