Hydrolyze Definition
To hydrolyze a bond is to break it apart with water. From the Greek words hydro and lysis, or “water break”, hydrolyze is literally just that. Water (or H2O) breaks into two parts: a positive hydrogen, H+, and a negative hydroxide, (OH)–. These charged molecules are used to split larger molecules by means of attracting different parts of a bond. By doing this a bond can be split, the hydroxide bonding to one half and the positive hydrogen to the other.
While there are a number of chemical reactions outside of biology that involve hydrolysis, there are many biological reactions that require water to hydrolyze the bonds of large molecules. Animals require water to hydrolyze sugars, lipids, and proteins. In other words, hydrolysis allows us to digest everything we eat. The following are some examples.
Examples of Hydrolyze
Protein Hydrolysis
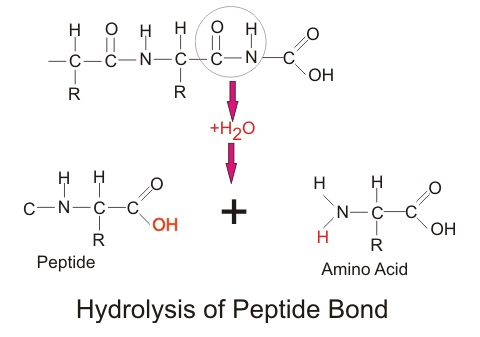
Everything we eat has a large amount of protein. Proteins are large molecules, and they are used by all forms of life to carry out a number of tasks. They are made up of many amino acids, connected together by peptide bonds. While our body can use the individual amino acids to make new proteins, it must break the amino acids apart first.
A peptide bond is formed between a nitrogen and carbon of two different amino acids. To break this bond apart, the more negative hydroxide bonds with the more positive carbon, while the hydrogen from the water binds with the more negative nitrogen of the second amino acid. Oftentimes living organisms use enzymes, or other proteins, to increase the speed and efficiency of the reactions. Interestingly, peptide bonds are created in the exact opposite way, by allowing the carbon and nitrogen to react and excluding a water molecule. This is known as a dehydration reaction.
When we hydrolyze the bonds in any protein, the protein is broken down into the individual amino acids that it is composed of. These amino acids are then used by your body to create the proteins it needs to function. In this way, the protein from a carrot can become the protein that makes up your muscle. All life on Earth originates from the same DNA, and all life on Earth uses essentially the same 20 amino acids. In fact, because we can hydrolyze protein into its basic parts, it is possible to get all the protein one needs from a diet consisting entirely of plants. The ‘fact’ that meat is somehow a requirement for protein is a common misconception.
Carbohydrate Hydrolysis
While our bodies use proteins for a number of purposes, we also need an energy source for our cells to do work. We mainly get this energy in the form of glucose. However, almost none of the foods that we eat contain glucose. Rather, they contain large polysaccharides that we must break down, or hydrolyze, into glucose. These polysaccharides are just long chains of glucose, used to store energy in a compact form. Also known as starches and complex sugars, these polysaccharides are the main way plants store the energy they harvest from the sun.
To hydrolyze these sugars and starches back into glucose, water is needed. However, simply putting a starch in water does not convert it to glucose at a rate that would help a living animal survive. To speed up the process another enzyme is used. This enzyme is a slightly different shape than the one used to hydrolyze amino acids, because sugars are a different shape than proteins. Once a cell has made the proper enzymes, the reaction can proceed very quickly. In fact, this process starts as soon as food enters the body. There are enzymes released in your saliva that quickly start to digest starches into more simple sugars. By getting glucose to the cells quickly, the cells can go through respiration and store the needed energy to process and incorporate the incoming amino acids and lipids from the meal. A well-balanced meal provides both energy and more resources to build and repair cells.
Related Biology Terms
- Dehydration Reaction – The opposite of hydrolyze, in which a water molecule is produced when two molecules are combined.
- Peptide Bond – A bond formed between two amino acids, used to make long chains of amino acids, a.k.a. proteins.
- Polysaccharide – A chain of several monosaccharides, typically glucose, created through dehydration reactions.
- Enzyme – A protein used to provide energy to a reaction and in doing so increase the speed of the reaction.
Quiz
1. A phospholipid is a large molecule made of smaller parts. The head of the molecule contains a phosphorous atom, and is attracted to water. The tail of the phospholipid contains long chains of carbons connected to hydroxyl groups. The tail is repelled by water. In this way phospholipids are used to create the cell membranes of all life on Earth. If a larger cell phagocytizes, or eats, another cell, what must it do to the phospholipids to digest them?
A. Dehydrate the bonds
B. Hydrolyze the bonds
C. Convert the bonds
2. In the reaction from the previous question, suppose the first bond the cell will hydrolyze is one between a more positive atom X and more negative atom Y. To which atoms will the hydrogen and hydroxide groups bond to?
A. Atom X to Hydrogen (H+) and Atom Y to Hydroxide ((OH)–)
B. Atom Y to Hydrogen (H+) and Atom X to Hydroxide ((OH)–)
C. Atom X to Hydrogen (H+) and Atom X to Hydroxide ((OH)–). Atom Y does not bond.
3. While animals are only consumers of glucose, plants are able to create glucose and use glucose. They create glucose from carbon dioxide, sunlight, and water, and use glucose through the same process of respiration that animals use. Therefore, glucose must be both dehydrated to be stored and hydrolyzed to be used. How can both of these processes happen at the same time?
A. They can’t. The plants must do them at different times.
B. They are separated by space.
C. The enzymes automatically turn on and off.