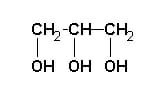Glycerol Definition
Glycerol is a colorless, odorless liquid with a sweet taste. It is viscous at room temperature and non-toxic in low concentrations. Glycerol was discovered in 1779. It is also called glycyl alcohol, glycerin or glycerine in some literature.
Glycerol is seen in biological systems as an intermediate in carbohydrate and lipid metabolism because surplus carbohydrate can be converted into long chain fatty acids and esterified with the three hydroxyl groups. Glycerol can influence immune reactions in the body through histamines, increased antibody production and by enhancing immune cell activity and is therefore classified as an allergen. In the blood, glycerol can increase blood pressure by preferentially attracting the water from tissues into plasma and lymph. In nephrons, glycerol can increase urine volume by preventing water resorption.
History of Glycerol
Glycerol was accidentally discovered by a Swedish scientist named K. W. Scheele. He was investigating the similarities between soap and a drying plaster called Emplastrum simplex. The salve was made of lead salts of fatty acids, while soap is made of sodium salts of organic acids. During his experiments of reacting olive oil with lead monoxide, he discovered a water-soluble substance with a sweet taste. This was the first recorded chemical isolation of glycerol and was initially called the ‘sweet principle of fat’. Scheele analyzed the substance and found it to be distinct from the other sugars known at the time. Glycerol did not crystallize, ferment, and showed greater heat resistance than most other sugars. He also investigated the difference between glycerol and cane sugar, especially in the proportion of oxygen (or phlogiston as it was then called) it contained. Scheele demonstrated that it took a greater amount of nitric acid to oxidize glycerol than cane sugar. It also did not release an alkali when it was reacted with ethanol. While it could not be easily crystallized, it could be distilled. It also decomposed at higher temperatures.
In 1836, the chemical formula of glycerol was elucidated by a French scientist called Pelouze. He proposed an empirical formula of C3H8O3. Fifty years later, the structural formula of C3H5(OH)3 was accepted, based on the work of two scientists named Berthelot and Lucea.
The relevance of glycerol as a commercially important chemical is linked to its use in the production of dynamite. Alfred Nobel, who later instituted the Nobel Prizes, discovered a method for the reliable stabilization, transport and handling of trinitroglycerin, which is the central explosive compound in dynamite. Glycerol, therefore, was involved in the rapid extraction of mineral ore, as well as many large-scale infrastructure projects that needed natural structures to be blasted away.
Properties of Glycerol
Pure glycerol has a melting point of 17.8°C. Its boiling point is 290°C but it also decomposes at that temperature. The presence of three hydroxyl groups makes the compound hygroscopic, with a tendency to absorb moisture from the air. This also makes it useful as a humectant in cosmetics and food, retaining water and preventing the substance from drying out.
Glycerol is easily soluble in water, due to the ability of the polyol groups to form hydrogen bonds with water molecules. Glycerol is slightly denser than water with a specific gravity of 1.26. This means that when glycerol is poured into a container of water, it will sink to the bottom. However, due to its solubility, over time and with mild agitation, glycerol will form an aqueous solution.
Glycerol can cause mild irritation to the eyes, nose, lungs and skin, particularly due to its hygroscopic nature. Skin and other internal organs can get dried out when pure glycerol comes into contact with these moist tissues. Since the molecule can bind to water, the same property that makes glycerol a good humectant also desiccates internal tissues. On the other hand, if a cosmetic preparation with high water content is applied on the skin, especially in arid environments, the presence of glycerol can prevent the lotion, cream or gel from drying out quickly.
The three hydroxyl groups of glycerol allow reactions with many organic acids to form esters. When all three reactive groups are esterified with long chain organic fatty acids, a triglyceride is formed. Triglycerides are among the most common lipids in the human body.
Uses of Glycerol
Glycerol is used in a number of industrial applications, in the pharmaceutical industry, in cosmetics and personal care products, in the production of resins, detergents, plastics and tobacco and as a humectant in food.
Its use as a commercially important chemical began with its application in the production of dynamite. Dynamite was necessary in the discovery and extraction of underground minerals, and in the construction of infrastructure. Therefore, it propelled industrial development.
Cosmetics and Food
Glycerol is used in the cosmetics industry as a moisture-control reagent and to enhance the texture of lotions and creams. Glycerol’s ability to retain moisture and its emollient properties make it an attractive ingredient in many moisturizing formulations. Glycerol can also prevent the cosmetic from either drying out or freezing.
In food, the utility of glycerol arises from its ability to form inter-molecular hydrogen bonds, especially with water molecules. This increases the water content in preserved food, without compromising on shelf life, and also enhances viscosity and texture. Its low toxicity and lack of a disagreeable odor or flavor allow the use of glycerol as an emulsifier.
Industrial Applications
Crude glycerin is a byproduct of the production of biofuels from soya bean oil and other vegetable oils. It contains over 60% impurities in the form of methanol, soaps and salts, making it difficult to extract pure glycerin. Recent advances in technology allow the use of crude glycerin to make urethane foams. Polyurethane foams have a variety of applications in the construction and automotive industries. They are also commonly used as insulators.
Pure glycerol is a crucial part of the industrial production of antifreeze, textiles and waxes. It is used in large quantities to generate resins, paints and waxes, for creating cleaning and purifying agents for soldering, and in the manufacture of many textiles and cosmetics.
Pharmaceuticals
Glycerol usage in the pharmaceutical industry is to improve smoothness and taste. It is used in the creation of tablets so that they are easy to swallow. The coating can disintegrate within the body. Cough lozenges often use glycerol to give a sweet taste. Suppositories of glycerol can act as laxatives since they can irritate anal mucosa.
Production of Triacetin
Triacetin is a triple ester of glycerol, formed through an esterifying reaction with acetic acid. It has a variety of uses in the food industry as a plasticizing agent, to enhance the viscosity of a product. It can also act as a stabilizer for food products that need to be preserved for extended periods of time.
Triacetin is used as an antiknock reagent in fuels for internal combustion engines. It is also an additive in cigarettes.
Glycerol Structure
Glycerol is a trihydroxy sugar alcohol with three carbon atoms and three hydroxyl groups. The presence of multiple hydroxyl groups and carbon atoms makes it an organic polyol compound with the IUPAC name of 1, 2, 3 – Propanetriol.
The structure of glycerol can be represented in a number of ways.
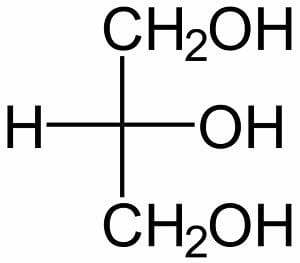
The simplest is the image above, showing the basic backbone of three carbon atoms, each of them covalently bonded to a hydroxyl group. Alternately, the molecule can be represented as a Fischer projection, centered on the second carbon atom, as seen in the image below.
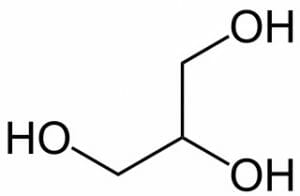
In addition, the molecule can be shown with a more accurate depiction of bond angles, without the explicit representation of the hydrogen atoms.
Quiz
1. What is the melting point of glycerol?
A. 290 °C
B. 17.8 °C
C. 1.26 °C
D. 3 °C
2. How is crude glycerin most commonly generated?
A. Byproduct of biofuel production
B. Byproduct of the pharmaceutical industry
C. End result of dynamite production
D. All of the above
3. Which of these are useful properties of glycerol?
A. It is hygroscopic in nature
B. It has three reactive oxygen molecules
C. It is mostly insoluble in water
D. All of the above
References
- Katryniok, B., et al., (2010). “Glycerol dehydration to acrolein in the context of new uses of glycerol”Green Chem.12:2079-2098.
- Gupta, M. and Kumar N., (2012) “Scope and opportunities of using glycerol as an energy source” Renewable and Sustainable Energy Reviews 16(7): 4551-56
- The National Institute for Occupational Safety and Health (2016). Glycerine: (mist) Retrieved 2017-04-15 from https://www.cdc.gov/niosh/npg/npgd0302.html
- The Soap and Detergent Association (1990). Glycerine: An Overview Retrieved 2017-04-15 from http://www.aciscience.org/docs/Glycerine_-_an_overview.pdf