Definition
Glial cells, neuroglial cells, or glia are no longer considered to have a purely structural role within the central nervous system; they have also been found to regulate nerve firing rates, brain plasticity, and immune responses. These numerous small cells that lack axons and/or dendrites have been the subject of significant research, but we are still only scratching the surface of many of their roles. Glial cells exist in the central nervous system (CNS) and the peripheral nervous system; some glial cell types can even move across the barrier between the CNS and PNS.
Glial Cells Function
The function of glial cells was previously thought to be a structural one, which is why they were named glia (Greek for glue) by nineteenth-century scientists. This function is now considered to be a minor one.
Glial cells function as modulators of the CNS and PNS environments; they increase and decrease activity within the synapses by regulating neurotransmitter, oxygen, and ion uptake; they also aid nerve injury recovery. Specific roles are carried out by the different glial cell types.
Glial cell abnormalities are associated with various pathologies including autoimmune disorders and cancer. This supports research that shows certain glia types are heavily involved in the immune system and inflammatory responses.
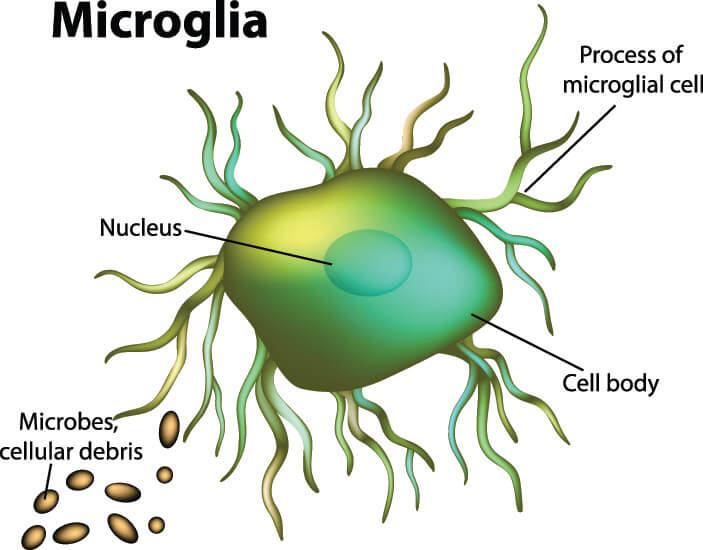
The macroglia (larger glial cells) insulate, protect, and help neurons to develop and migrate. Microglia (smaller types of glia) have phagocytic properties, digesting foreign particles. They even contribute to nervous tissue immune responses and repair processes as they can migrate to areas of damage. Furthermore, micro- and macroglia work together to ensure optimal neuron function and neurotransmission through the synapses.
The role of glial cells in the field of human psychology is also becoming apparent. For example, postmortem results of people affected with bipolar disorder show much lower glial cell populations in specific areas of the brain. In living bipolar patients, microglia overactivity – maybe compensating for lower numbers of cells – has been noted. This loss or overactivity of glial tissue may be an important contributing factor to nerve degeneration in associated regions of the brain. Glial cells certainly play a role in modulating the effects of the mood-stabilizing drugs used to treat bipolar disorder.
Glial Cell Types
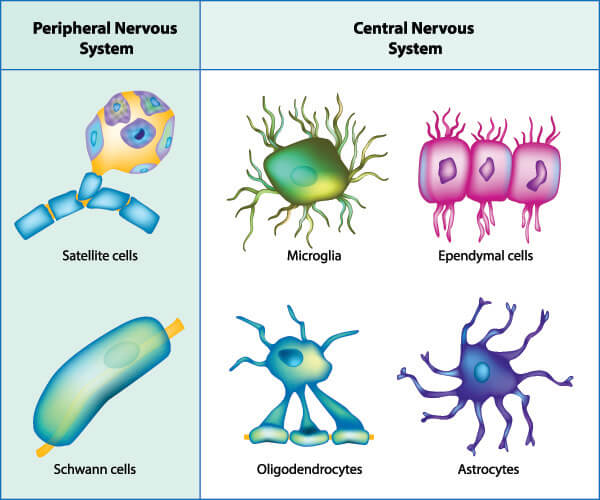
Glial cell types all stem from two major categories – the macroglia and microglia. While macroglia are involved in regulating and optimizing nerve cell function, microglia make the immediate environment safer. Macroglia and macroglia (or their sub-types) are found in both the CNS and PNS and present with specific but often overlapping or collaborative roles.
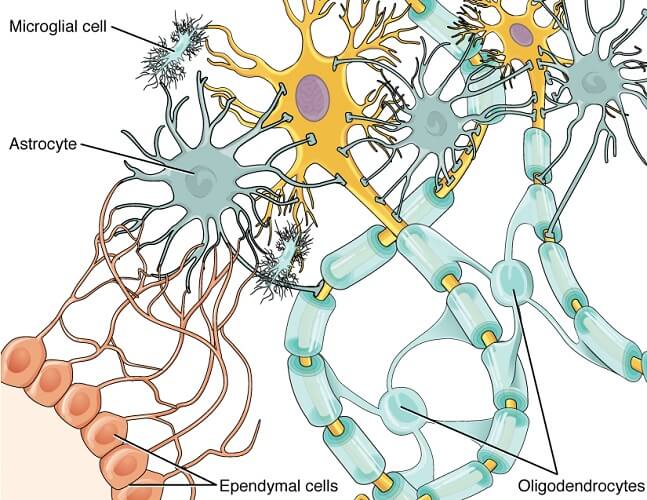
Macroglia are found in seven different forms spread throughout the entire nervous system. These are oligodendrocytes, astrocytes, ependymal cells, radial glia, Schwann cells, satellite cells, and enteric glia. We will look at these in more detail further on. Microglia were, until very recently, thought to be limited to the central nervous system. They were then seen to cross into the peripheral nervous system in zebrafish, where they would pick up cellular debris from places of nerve fiber injury. The microglia would then return to the brain still holding this debris but the cells would become altered in the process. As altered microglial cells are found in large quantities in human neurodegenerative disease, this migration and return probably occurs in higher species, too.
Macroglia in the CNS
Macroglia in the CNS are grouped into subcategories of ependymal cells, oligodendrocytes, radial glia, and astrocytes.
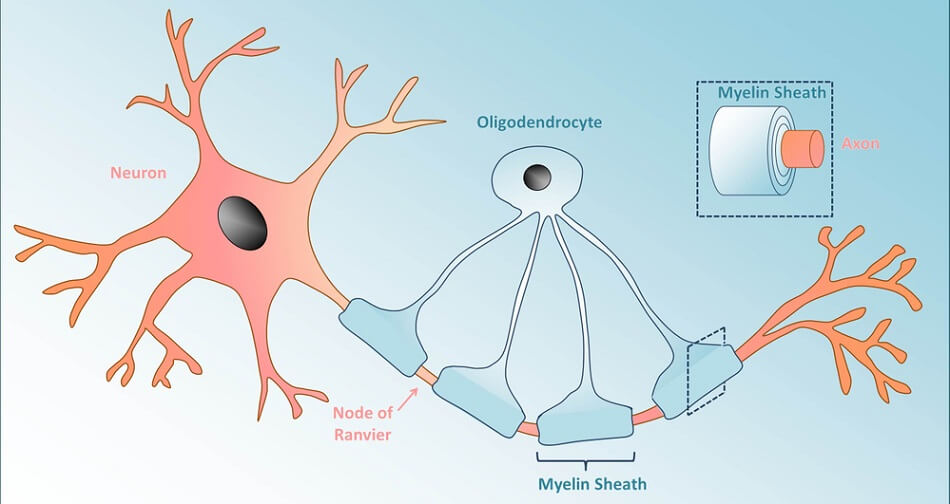
Oligodendrocytes are best known for their ability to manufacture, repair, and arrange myelin sheaths around neuron axons. Myelin sheaths insulate nerve cell axons to prevent electrical impulses from leaking and enabling longer-distance communication. Oligodendrocytes also support the metabolic needs of the nerve cell axon.
Astrocytes are divided into star-like fibrous astrocytes and protoplasmic astrocytes, both of which connect signal-producing tissues (neurons) to cells that do not have this mode of communication, like blood vessels. Astrocytes also help to maintain the permeability of the blood-brain barrier where they sense glucose and ion levels inside the brain and regulate their flow into or out of it.
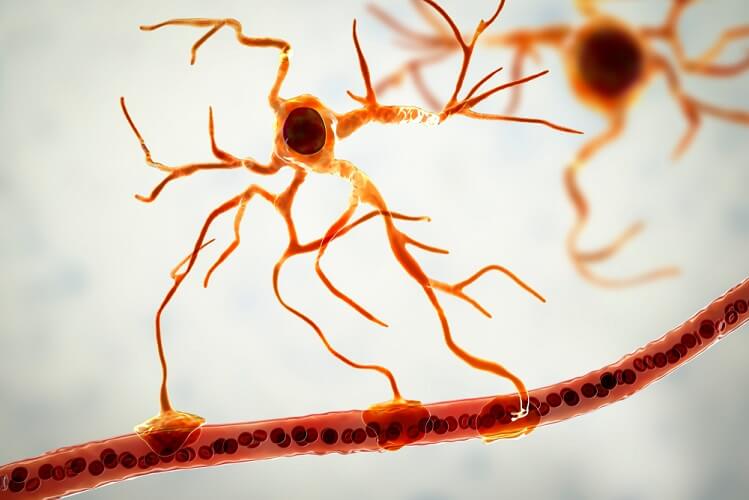
Radial glia are only found in specific areas of the CNS. This subgroup includes the Bergmann and Müller cells of the cerebellum and retina respectively. Radial cells modulate neurotransmission and optimize how information is processed. By forming a framework or scaffold on which other neurons can travel, radial glial cells are highly communicative. They also play roles in ion homeostasis, increased synapse stability, and improved brain plasticity and neuroprotection. This is done by regulating the surrounding extracellular fluid.
Müller cells act as optical fibers that guide incoming light through the retina to minimize scattering; this makes for a clearer image. At the same time, they surround neurons and stabilize and protect the nervous tissue of the back of the eye. And, like all radial glia, they simultaneously regulate ions and glucose in the extracellular space.
Ependymal cells (ependymocytes) line the brain ventricles and spinal cord canal in a continuous sheet of epithelium known as the ependyma. These cells primarily produce cerebrospinal fluid (CSF). Depending on where they are located, ependymal cells also help to distribute neurotransmitters and hormones associated with the central nervous system. Furthermore, the microvilli of ependymal cells can absorb CSF and influence its flow and let certain substances in and out of the brain. Like the majority of glial cells, the ependyma also contributes to osmotic control within the brain via glucose and ion regulation.
Macroglia in the PNS
Macroglia contained in the peripheral nervous system are satellite glial cells, Schwann cells, and enteric glia.
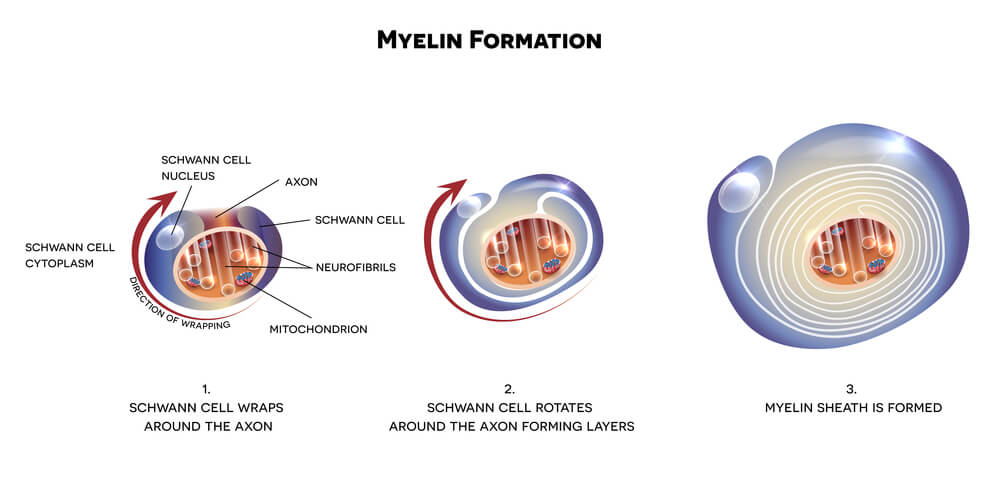
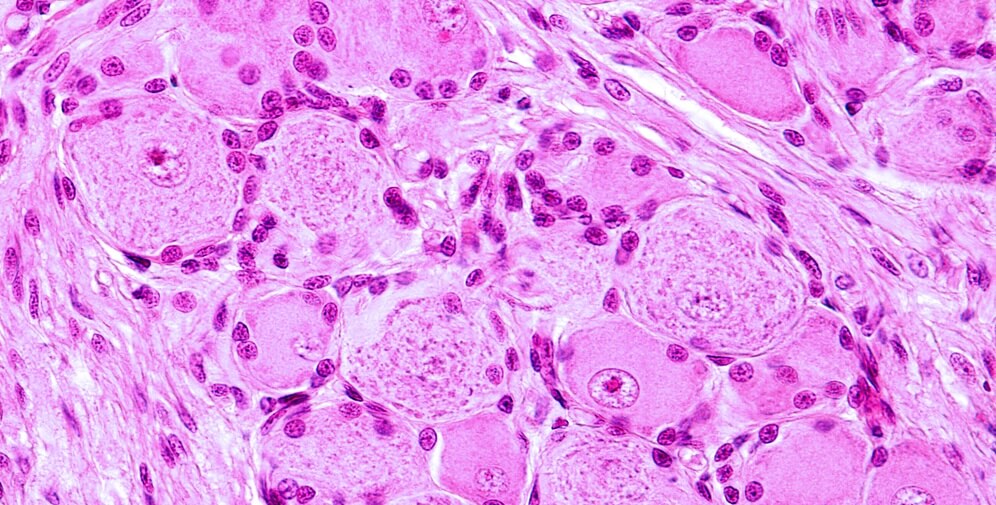
Schwann cells (neurolemma) of the PNS mirror the role of oligodendrocytes in the central nervous system; they myelinate the axons of neurons and modulate extracellular fluid. However, while a single oligodendrocyte will provide insulation for multiple neurons in the CNS, the opposite is true in the PNS – a single axon hosts multiple Schwann cells, each of which myelinates its own section. The picture shows the tree-ring form of the myelin layers that surround the central axon of the nerve cell.
Satellite glial cells or SGCs surround the sensory and autonomic ganglia. Ganglia are relay stations where one nerve enters and another exits. In autonomic (involuntary) nervous system pathways, SGCs respond to chemical messengers (neurotransmitters) and optimize them so that vital responses such as heart rate and vasoconstriction go as smoothly as possible. In the sensory nervous system, satellite cells regulate potassium levels and the neurons’ response to evoked potentials without the presence of neurotransmitters. Charcot-Marie-Tooth disease (CMT) describes a group of inherited conditions that cause damage to the peripheral nerves, often specifically to SGCs. Symptoms include impaired sensory and motor activity of the limbs that causes mild to severe loss of sensation and muscle weakness. Satellite glial cells are also associated with chronic and acute pain responses.
Enteric glia are found in the lining of the intestines. They assist with gut motility (peristalsis) and enable contact between different cells of the intestinal wall. The enteric nervous system or ENS is secondary in cell population only to the central nervous system. Enteric glial cells seem to feature characteristics of other nervous tissue cells such as astrocytes and oligodendrocytes. Recent topics such as the gut-brain axis will undoubtedly throw more light on the importance of enteric glia. The fact that these cells are more broadly differentiated than any other type of glial cells makes them interesting subjects. What is already known is that enteric glial cells change in function and form according to their location, the age of the host, and even that person’s gender.
Microglia in the CNS
Microglia in the CNS are not totally limited to the central nervous system – they like to take short trips into peripheral nerve tissue. Researchers have found that they temporarily migrate into the peripheral nervous syste in rodents and zebrafish, and the presence of slightly changed microglia in human brain degeneration tells us that this occurs in humans, too. Microglia seem to change after they have traveled into the peripheral nervous system – imagine coming home with a sun tan or tummy bag after a trip away from home.
Microglia play important roles in nervous tissue immunity and inflammatory responses, working much like macrophages and clearing away cellular debris and toxins. Other roles include optimizing different brain circuits to enable cognitive development – microglia cells eliminate previously-formed synapses that are no longer useful. These cells can modulate the mechanisms of memory and learning, and their degeneration has been associated with Alzheimer’s disease.
Glial Cells vs Neurons
Glial cells versus neurons articles always begin with the same description – glia do not transmit impulses but neurons do. However, current studies show that this is not entirely true. While it is true that glial cells do not have synapses, they can communicate with neurons and create an environment in which synapses can be inhibited or fired up. This interaction is called the tripartite synapse, the communication that crosses between glial cells, presynaptic neurons, and postsynaptic neurons. The synapse most often described in the average textbook is the bipartite synapse that exists only between the pre- and postsynaptic neurons and ignores the roles of glial cells.
Glial cells exist in significantly more numbers than neurons but do not have axons. Furthermore, mature and differentiated glial cells can divide by mitosis; neurons only undergo cell division if they have not yet differentiated into their final form. Because glial cells are also structural – although this is not their main role as previously thought – the ability to divide and multiply is essential. A strong nervous tissue structure also ensures optimal conditions for sending messages throughout the body. All of these additional functions offer a potential explanation for why glial gene mutations are found in some psychological and neural disorders. This is definitely the case in Huntingdon’s disease.