Electron Transport Chain Definition
The electron transport chain is a cluster of proteins that transfer electrons through a membrane within mitochondria to form a gradient of protons that drives the creation of adenosine triphosphate (ATP). ATP is used by the cell as the energy for metabolic processes for cellular functions.
Where Does the Electron Transport Chain Occur?
During the process, a proton gradient is created when the protons are pumped from the mitochondrial matrix into the intermembrane space of the cell, which also helps in driving ATP production. Often, the use of a proton gradient is referred to as the chemiosmotic mechanism that drives ATP synthesis since it relies on a higher concentration of protons to generate “proton motive force”. The amount of ATP created is directly proportional to the number of protons that are pumped across the inner mitochondrial membrane.
The electron transport chain involves a series of redox reactions that relies on protein complexes to transfer electrons from a donor molecule to an acceptor molecule. As a result of these reactions, the proton gradient is produced, enabling mechanical work to be converted into chemical energy, allowing ATP synthesis. The complexes are embedded in the inner mitochondrial membrane called the cristae in eukaryotes. Enclosed by the inner mitochondrial membrane is the matrix, which is where necessary enzymes such as pyruvate dehydrogenase and pyruvate carboxylase are located. The process can also be found in photosynthetic eukaryotes in the thylakoid membrane of chloroplasts and in prokaryotes, but with modifications.
By-products from other cycles and processes, like the citric acid cycle, amino acid oxidation, and fatty acid oxidation, are used in the electron transport chain. As seen in the overall redox reaction,
2 H+ + 2 e+ + ½ O2 → H2O + energy
energy is released in an exothermic reaction when electrons are passed through the complexes; three molecules of ATP are created. Phosphate located in the matrix is imported via the proton gradient, which is used to create more ATP. The process of generating more ATP via the phosphorylation of ADP is referred to oxidative phosphorylation since the energy of hydrogen oxygenation is used throughout the electron transport chain. The ATP generated from this reaction go on to power most cellular reactions necessary for life.
Steps of the Electron Transport Chain
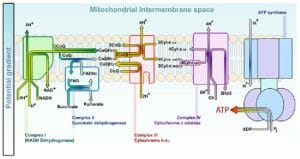
In the electron transfer chain, electrons move along a series of proteins to generate an expulsion type force to move hydrogen ions, or protons, across the mitochondrial membrane. The electrons begin their reactions in Complex I, continuing onto Complex II, traversed to Complex III and cytochrome c via coenzyme Q, and then finally to Complex IV. The complexes themselves are complex-structured proteins embedded in the phospholipid membrane. They are combined with a metal ion, such as iron, to help with proton expulsion into the intermembrane space as well as other functions. The complexes also undergo conformational changes to allow openings for the transmembrane movement of protons.
These four complexes actively transfer electrons from an organic metabolite, such as glucose. When the metabolite breaks down, two electrons and a hydrogen ion are released and then picked up by the coenzyme NAD+ to become NADH, releasing a hydrogen ion into the cytosol.
The NADH now has two electrons passing them onto a more mobile molecule, ubiquinone (Q), in the first protein complex (Complex I). Complex I, also known as NADH dehydrogenase, pumps four hydrogen ions from the matrix into the intermembrane space, establishing the proton gradient. In the next protein, Complex II or succinate dehydrogenase, another electron carrier and coenzyme, succinate is oxidized into fumarate, causing FAD (flavin-adenine dinucleotide) to be reduced to FADH2. The transport molecule, FADH2 is then reoxidized, donating electrons to Q (becoming QH2), while releasing another hydrogen ion into the cytosol. While Complex II does not directly contribute to the proton gradient, it serves as another source for electrons.
Complex III, or cytochrome c reductase, is where the Q cycle takes place. There is an interaction between Q and cytochromes, which are molecules composed of iron, to continue the transfer of electrons. During the Q cycle, the ubiquinol (QH2) previously produced donates electrons to ISP and cytochrome b becoming ubiquinone. ISP and cytochrome b are proteins that are located in the matrix that then transfers the electron it received from ubiquinol to cytochrome c1. Cytochrome c1 then transfers it to cytochrome c, which moves the electrons to the last complex. (Note: Unlike ubiquinone (Q), cytochrome c can only carry one electron at a time). Ubiquinone then gets reduced again to QH2, restarting the cycle. In the process, another hydrogen ion is released into the cytosol to further create the proton gradient.
The cytochromes then extend into Complex IV, or cytochrome c oxidase. Electrons are transferred one at a time into the complex from cytochrome c. The electrons, in addition to hydrogen and oxygen, then react to form water in an irreversible reaction. This is the last complex that translocates four protons across the membrane to create the proton gradient that develops ATP at the end.
As the proton gradient is established, F1F0 ATP synthase, sometimes referred to as Complex V, generates the ATP. The complex is composed of several subunits that bind to the protons released in prior reactions. As the protein rotates, protons are brought back into the mitochondrial matrix, allowing ADP to bind to free phosphate to produce ATP. For every full turn of the protein, three ATP is produced, concluding the electron transport chain.
Quiz
1. Complex IV, also known as cytochrome oxidase, performs which reaction?
A. NADH + Q ↔ NAD+ + QH2
B. NADH ↔ NAD+ + 2H+ + 2e–
C. 2 H+ + 2 e+ + ½ O2 → H2O + energy
D. 4 H+ + 4 e– + O2 → 2 H2O
2. What component(s) is passed to the first complex in the electron transport chain?
A. NADH + H+
B. FADH+
C. Q
D. Cytochrome c
3. Where is the higher concentration of protons while the electron transport chain is activated?
A. Phospholipid layer
B. Mitochondrial matrix
C. Intermembrane space
D. Cell membrane