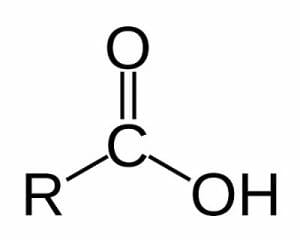
Carboxyl Group Definition
A carboxyl group is one of many functional groups that attaches to larger molecules and gives them certain properties. The carboxyl group is seen in many organic molecules known as carboxylic acids, which have a variety of functions. The carboxyl group consists of a carbon, bonded to both an oxygen and a hydroxyl group. Hydroxyl groups are simply an oxygen bonded to a hydrogen. The structure of a carboxyl group can be seen below.
The double-bonded oxygen is electronegative, and attracts hydrogens. The hydroxyl group does the opposite, and would gladly give up a hydrogen to form another bond with carbon. In this way, carboxyl groups are polar, and can participate in hydrogen bonding and a variety of other important reactions. The “R” in the above diagram can be any number of carbon-containing molecules, or even a single hydrogen atom. One important example of a carboxyl group is in protein synthesis. Every amino acid has both a carboxyl group and an amino group. The bond formed between these groups allows amino acids to be chained together in long sequences, and is known as a peptide bond. Carboxyl groups are attached to a large variety of other molecules and serve a number of roles in biology.
Related Biology Terms
- Functional Group – A functionally significant section of a molecule, with specific chemical properties.
- Hydroxyl Group – An oxygen bonded to a hydrogen, which can exist freely in solution or attached to a molecule.
- Carboxylic Acid – A large group of carboxyl group based organic molecules which can donate the proton of the hydroxyl group to a number of reactions.
Quiz
1. One important use of the carboxyl group for a certain group of ants is formic acid. Formic acid is simply a carboxyl group attached to a hydrogen. The ants inject this acid into enemies as a defense. Why does this work?
A. The carboxyl group forms bonds with their proteins
B. The acid upsets the pH balance, and destroys cells
C. The acid simply tastes bad to predators