Biogeochemical Cycle Definition
A biogeochemical cycle is one of several natural cycles, in which conserved matter moves through the biotic and abiotic parts of an ecosystem.
In biology, conserved matter refers to the finite amount of matter, in the form of atoms, that is present within the Earth. Since, according to the Law of Conservation of Mass, matter cannot be created or destroyed, all atoms of matter are cycled through Earth’s systems albeit in various forms.
In other words, the Earth only receives energy from the sun, which is given off as heat, whilst all other chemical elements remain within a closed system.
The main chemical elements that are cycled are: carbon (C), hydrogen (H), nitrogen (N), oxygen (O), phosphorous (P) and sulfur (S). These are the building blocks of life, and are used for essential processes, such as metabolism, the formation of amino acids, cell respiration and the building of tissues.
These fundamental elements can be easily remembered with the acronym CHNOPS.
Each of these elements is circulated through the biotic components, which are the living parts of an ecosystem, and the abiotic components, which are the non-living parts.
The abiotic components can be subdivided into three categories: thehydrosphere (water), the atmosphere (air) and the lithosphere(rock).
The biosphere is a term which can be used to describe the system that contains all living organisms, including plants, animals and bacteria, as well as their interactions among and between each other, and their interactions with the Earth’s abiotic systems. The biosphere is sometimes called the ecosphere, and can be defined as the sum of all ecosystems.
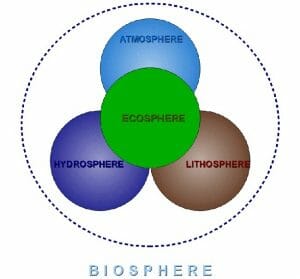
With this knowledge, the words “biogeochemical cycle” can be easily broken down. “Bio-” is the biotic system, “geo-” is the geological component, and “chemical” is the elements which are moved through a “cycle”.
At particular stages of their cycling, any of the elements may be stored and accumulated within a particular place for a long period time (e.g. within a rocky substrate, or in the atmosphere). These places are called “sinks” or “reservoirs”.
A “source” is anything from which an element is output, for example volcanoes give off large amounts of carbon in the form of CO2, while human waste is a source for nitrogen, sulfur and phosphorous.
Examples of Biogeochemical Cycle
The Water Cycle
The biogeochemical cycle of water, or the hydrological cycle describes the way that water (Hydrogen Dioxide or H2O) is circulated and recycled throughout Earth’s systems.
All living organisms, without exception, need water to survive and grow, making it one of the most important substances on Earth. In complex organisms it is used to dissolve vitamins and mineral nutrients. It is then used to transport these substances, as well as hormones, antibodies, oxygen and other substances around and out of the body. It also aids in the enzymatic and chemical reactions required for metabolism, and it is used for temperature regulation.
On a geographical level, the biogeochemical cycle of water is responsible for weather patterns. The temperature, the amount, and the movement of water, have an effect all weather systems. As water in its various forms (vapor, liquid and ice) interacts with its surroundings, it alters the temperature and pressure of the atmosphere, creating wind, rain and currents, and is responsible for changing the structure of earth and rock through weathering.
Although there is no real beginning to the water cycle, 97% of the world’s water is stored within the oceans, so here is a logical place to start.
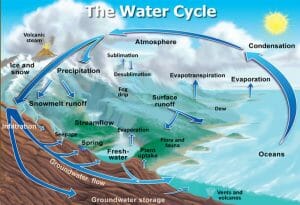
Of the ocean water, a very small proportion becomes frozen at it reaches the poles, and is stored as ice within glaciers.
Some of the surface water is heated by the sun, and evaporation takes place. In this process, the liquid water is converted into water vapor and is taken up in to the atmosphere. As the water rises, it cools and condensation occurs. This results in the water being stored within the atmosphere in the form of clouds.
As the clouds are moved around the earth’s atmosphere they collide and grow. Eventually the water droplets grow large enough so that they are heavy enough to fall as precipitation (rain) or as snow, depending on the environmental conditions.
Most of the snow that falls is either stored as ice caps, or melts to form streams and rivers.
Some of the water that makes it to the ground is affected by gravity and flows back in to the ocean via surface runoff. Furthermore some of this water joins with freshwater streams and rivers, which eventually lead to the oceans, or it may be stored within lakes and reservoirs. This freshwater can be consumed by animals, who cycle the water through their bodies.
Much of the water that fell as rain, soaks in to the ground through infiltration. Here it either infiltrates deep into the rock, and forms huge stores called aquifers or it remains relatively close to the surface as groundwater flow.
The groundwater is taken in by the roots of plants and is used for photosynthesis. The water is then released into the atmospheric through evapotranspiration or is consumed when the plants are eaten.
Some of the groundwater emerges from springs and surface water bodies, eventually making its way back to the ocean.
The Carbon Cycle
As a main component of biological compounds, carbon can be found in all living things, as well as many non-living things such as minerals, the atmosphere, the oceans and the interior of the earth.
Although carbon is an essential component for life, it is only due to a specific balance of atmospheric components and conditions that life, as we know it, is able to exist. Therefore, it is important that a balance between the amount of carbon stored in sinks and the amount that is emitted from various sources is maintained.
Although all biogeochemical cycles of carbon are linked, it is simpler to vizualise them using two systems.
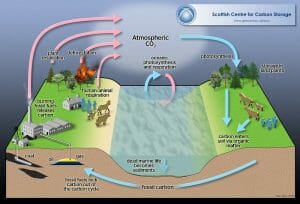
Rapid Carbon Biogeochemical Cycles
In this cycle, inorganic carbon, which is present in the atmosphere as CO2, is captured by autotrophs. These are usually photosynthesizing organisms such as plants, bacteria and algae.
During photosynthesis, the carbon is converted into organic compounds such as glucose, which are stored within the bodies of these organisms. This carbon can be stored for many hundreds of years within the bodies of plants in areas such as tropical rainforests.
When the organic compounds are consumed by heterotrophs, they are passed through the food web, where they are broken down into useful substances using cellular respiration. Cellular respiration produces CO2, which is released back into the atmosphere.
The ocean is the second largest carbon sink. As well as dissolved inorganic carbon which is stored at depth, the surface layer holds large amounts of dissolved carbon that is rapidly exchanged with the atmosphere.
Long-term Carbon Biogeochemical Cycles
The long-term storage of carbon occurs over thousands or millions of years and is important for maintaining stable atmospheric carbon levels.
When an organism dies, the carbon stored within their body is broken down into CO2 and other organic substances by decomposers. While some of this carbon is released into the atmosphere, a large portion of it remains sequestered within the soil. Through this process, soils become major reservoirs for carbon storage.
The largest carbon sink is the lithosphere (the earth’s rocks). Much of the earth’s carbon was stored within rocks when the earth was formed, however, it is also continuously cycled through the biogeochemical cycle of the biosphere. Calcium carbonate (CaCO3), which makes up the shells of marine organisms, forms limestone when it collects at the bottom of the ocean. This is one of the world’s largest carbon reservoirs.
Fossil fuels also contain huge amounts of carbon; these are formed from the remains of plants and animals that lived millions of years ago. Under specific conditions, the carbon within their bodies was pressurized and ‘cooked’ to form hydrocarbons. Today this is found in the form of crude oil, coal and natural gas.
Human Impacts on the Carbon Biogeochemical Cycle
Humans are having a drastic impact on the natural cycling of carbon in the atmosphere and in the oceans.
Fossil fuels, which have stored vast amounts of carbon for millions of years, are being burned at a rate that is too fast for it to be returned to carbon sinks. Instead it is being released into the atmosphere as carbon dioxide and methane (CO) which prevents heat from escaping the atmosphere, resulting in the greenhouse effect.
Additionally, among other disruptive practices, deforestation is releasing carbon stored within plant matter and is reducing the number of plants available to capture it – this is especially true in tropical rainforests and peat bogs.
The unnatural interference with this delicate biogeochemical cycle by humans could have severe consequences for our planet.
Related Biology Terms
- The Nitrogen Cycle – The biogeochemical cycle through which nitrogen is transferred through biotic and abiotic components of an ecosystem.
- The Energy Cycle – The cycle which describes the transfer of energy from the sun, through photosynthetic organisms, to heterotrophs and back out as heat.
- Elements – A substance, usually occurring naturally, that cannot be broken down into its simple atomic components.
- Abiotic factors – The non-living parts of an ecosystem that affect the biotic factors, for example, temperature, light and nutrients.
Quiz
1. A biogeochemical cycle describes the cycling of conserved matter through ______.
A. Rocks
B. Water
C. Plants
D. All of the above
2. Which of the following does not belong in the acronym CHNOPS?
A. Phosphorus
B. Helium
C. Sulfur
D. Oxygen
3. In the carbon cycle, what do plants capture in order to photosynthesize?
A. Hydrogen
B. Inorganic Carbon
C. Organic Carbon
D. Heat
