Autophagy Definition
Autophagy is the digesting of damaged cell organelles and diseased tissue for later recycling or repurposing. Its ancient Greek wording derives from the words for “hollow” and “self-devouring,” or “eating of self,” which accurately describes it as a natural way of pruning off dysfunctional parts. Autophagy is finely-controlled and targeted with the help of lysosomes (in animals) or other digestive factors that “eat” away at cellular components. These cellular components can take the form of organelles, protein aggregates or dysfunctional ribosome proteins (the protein makers of the body).
Autophagy is often said to be a self-degradative process and typically runs in response to stress or disease. The stress generally comes from a drive to survive in times of nutrient stress or energy depletion, or even from the cellular stress of experiencing illness or initiating cell death. One can say that autophagy plays a role in cell repair and detoxification. All of this can be finely targeted with the help of cell surface antigens, which lend autophagy a role in protecting against specific presentations of disease (i.e. cancer, diabetes, autoimmune infections) and genomic instability.
The picture depicts a simplified structure of Lysosome.
Autophagy Pathway
The basic path that autophagy follows begins with an isolated membrane called a phagophore. The phagophore expands and encircles the targets or “cell cargo” and sequesters them into its double membrane. The phagophore then fuses with a lysosome (in mammals, vacuoles in plants) that promotes the degradation of the components inside by lysosomal proteases. Amino acids and other by-products of this step exit into the cytoplasm, where they can help build new molecules later.
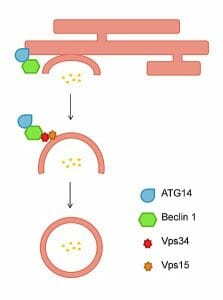
The figure depicts the vesicle nucleation of autophagosomes from an isolation membrane.
In technical terms, there are five stages that go into orchestrating autophagy.
- Phagophore forms
- Atg5-Atg12 proteins conjugate and interact with Atg16L inside the phagophore
- LC3 protein is modified and inserted into its membrane
- Targets are selected for and degraded (but random things can be taken up, as well)
- Autophagosome fuses with lysosome and the engulfed molecules are broken up
Phagophore formation begins with making an isolated membrane, a key step since the vesicle’s function relies on its ability to engulf contents. This process is organized around a structure called PAS (a pre-autosomal structure). In mammalian macroautophagy, the pathway autophagy takes begins with an “initiation” step with PAS. The phagophore then commences “nucleation,” which is basically the circling of cytosolic proteins and organelles. Next, the phagophore matures into an autophagosome with the addition of LC3 in the membrane, and begins “expansion.” The next step involves the fusing of a lysosome that contains hydrolases. As a result, the contents that were inside are broken into parts that can be recycled – a process termed “nutrient recycling.”
Another notable fact about autophagy is that it was previously thought to be only a pro-death mechanism. This misconception came from evidence that showed its involvement in non-apoptotic death. But recent research suggests it rather promotes life and survival than cell death. Autophagy occurs in all cells to upkeep basal, homeostatic conditions. Specifically, cells perform it to promote organelle turnover, especially when the cells are experiencing starvation and are in need of nutrients. Factors that play into the control of autophagy are nutrition and hormone levels, and external cues like oxygen levels and temperature. For instance, autophagy was exclusively thought to contribute to neurodegeneration, but recent research suggests it may be a protective way of degrading malfunctioning or toxic proteins in neural cells.
Autophagy Fasting
Autophagy has gained traction for its relationship to exercise, diet and fasting. All of these elements place stress on our bodies, which stimulates self-cleansing at the cellular level. With exercise, working out creates minute tears in our muscles that the body tends to immediately with autophagy and cell turnover and recycling. This strengthens the affected muscles and make them more resistant to future stressors. One study on mice showed that autophagosomes become increasingly numbered with thirty minutes of exercise on a treadmill (and even more so with eighty minutes). In humans, this relationship continues to be determined but certainly sets a precedent. Intermittent fasting holds similar benefits. Insulin levels increase after a meal in most model organisms, and there appear to be low rates of autophagy associated with high insulin. However, when insulin levels drop during fasting, autophagy rates increase many folds in mice (and theoretically in humans as well). High rates of autophagy are present in young organisms, but with aging, autophagy rates slow and this allows cell damage and toxicity to amass. Likewise, neuronal cells were studied in fasting mice to test the effect of short-term fasting on neurodegeneration. Fasting saw an increase in autophagosome count (via electron microscopy), and reduced phosphorylated purkinje ribosome as well. All of this attests to autophagy’s homeostatic role against degenerative pathways, which appears to be actively reset in times of fasting.
Quiz
1. Autophagy relies on the action of which organelle in mammals?
A. Peroxisomes
B. Mitochondria
C. Golgi bodies
D. Lysosomes
2. Correctly name the order of steps involved in macroautophagy.
A. Initiation, enucleation, shrinking, recycling
B. Initiation, nucleation, expansion, waste
C. Initiation, nucleation, expansion, recycling
D. Initiation, enucleation, dispersing, waste
3. With exercise/fasting, autophagy ______________.
A. Increases/decreases
B. Decreases/increases
C. Decreases/decreases
D. Increases/increases
References
- He C et al. (2012). “Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis.” Nature. 481(7382): 1476-4687.
- Glick, D et al. (2010). “Autophagy: cellular and molecular mechanisms.” J Pathol. 221(1): 3-12.
- Kaur, J and Debnath, J (2015). “Figure 1: Overview of mammalian autophagy pathways.” Nature Reviews Molecular Cell Biology. (16): 461-472. doi:10.1038/nrm4024
- Alirezaei M et al. (2010). “Short-term fasting induces profound neuronal autophagy.” Autophagy. 6(6): 702-710.
- Mangan P.D. (2016). “The Sweet Spot for Intermittent Fasting.” The mission Co. Retrieved 2017-05-25 from https://themission.co/the-sweet-spot-for-intermittent-fasting-9aae12a2158c
