AP Biology 3.1 - Enzyme Structure
Here, we take a look at section 3.1 of the AP Biology curriculum – Enzyme Structure. Since the large majority of enzymes are proteins, we’ll start by looking at protein structure. We’ll see how enzymes use an active site and other structures to function. Then, we’ll take a look at the specific properties of enzymes. We’ll start with the catalytic properties of enzymes that allow them to lower the activation energy of reactions. After that, we’ll look at enzyme specificity and reversibility and see how enzymes are highly sensitive to changes in the temperature and pH of the solution they are in.
Video Tutorial
The following video summarizes the most important aspects of this topic!
To watch more tutorial videos like this, please click here to see our full Youtube Channel!
Resources for this Standard
For Students & Teachers
- Overview & Video Tutorial (This article)
- Quick Test Prep
- Crossword Puzzle
For Teachers Only
ENDURING UNDERSTANDING
ENE-1
The highly complex organization of living systems requires constant input of energy and the exchange of macromolecules.
LEARNING OBJECTIVE
ENE-1.D
Describe the properties of enzymes.
ESSENTIAL KNOWLEDGE
ENE-1.D.1
The structure of enzymes includes the active site that specifically interacts with substrate molecules.
ENE-1.D.2
For an enzyme-mediated chemical reaction to occur, the shape and charge of the substrate must be compatible with the active site of the enzyme.
3.1 Enzyme Structure Overview
Sugars like glucose form the basis of all life on Earth because there is a lot of energy in the bonds of 1 sugar molecule. If you were to simply pour this sugar into water and wait – it would take thousands of years for all the energy to be slowly released from the bonds. But, when you eat sugar, the energy from this substance is available to your cells in a matter of minutes! This is because cells have a trick up their sleeves – enzymes!
Your body produces thousands of enzymes to not only break apart all types of macromolecules but also produces enzymes to put the pieces back together again! Enzymes are simply proteins with a specific structure that enables them to combine or break down substrate molecules. Since this will definitely be on the AP test, you should stick with us as we cover everything you need to know about Enzyme Structure and the Properties of Enzymes!
Besides a handful of RNA structures that can catalyze biochemical reactions and act as enzymes, the large majority of enzymes are simply proteins with very specific structures. So, let’s do a quick review of protein structure.
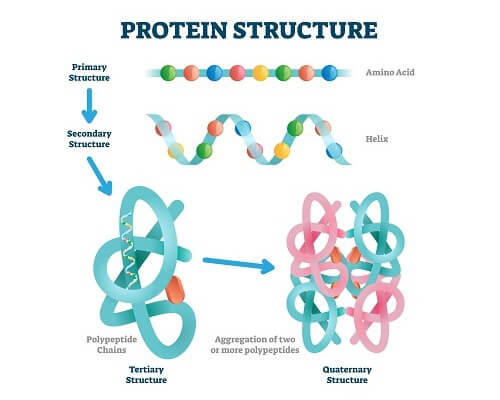
All proteins are simply a chain of amino acids. There are 20 different amino acids with specific properties that are connected together by ribosomes to create large chains. This chain of amino acids is known as primary structure.
Once the ribosome has attached the appropriate amino acids in the right order, the polypeptide naturally forms a secondary structure. This secondary structure is created by interactions between adjacent polypeptides, forming structures like alpha-helices and beta-sheets.
Now, the protein must enter the right environment to fold into a more complex tertiary structure. This structure is based on complex interactions between secondary motifs and individual amino acids. Finally, quaternary structure is formed when several individual polypeptide chains come together into a much larger protein structure.
The only real difference seen in enzyme structure is the active site. The active site is simply the place within the protein structure where a substrate molecule (or molecules) can bind. As the substrate binds to an enzyme, the enzyme changes shape slightly and applies a force to the substrate. This conformational change can either break a larger molecule apart or force two smaller molecules together! Some enzymes also have secondary sites where molecules like coenzymes can bind. These molecules allow enzymes to be turned on and off so the cell can control exactly when the enzyme is allowed to function.
Think about this… when you get a blood test from your doctor, one of the things they measure is the amount of different types of enzymes in your blood. Using this information, your doctor can infer the different types of reactions that are taking place within your body. Since enzymes are very specific to the substrate molecules that they act on, the presence of an enzyme in your bloodstream is proof that certain reactions are taking place in your body. Some enzymes are indicative of good health, while the presence of other enzymes can indicate that certain areas of your body are not working properly. As we continue into the properties of enzymes, think about how a doctor could use these properties to predict what is happening in your body!
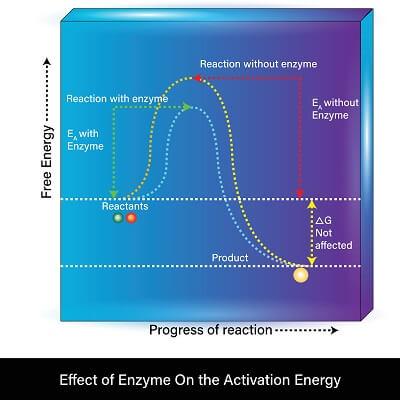
The most important property of enzymes is the catalytic property. In other words, enzymes are able to catalyze (or speed up) reactions by lowering the activation energy they need to get started.
Without an enzyme, most reactions require quite a bit of energy to get started. Since the enzyme changes shape once it is bound to the substrate, it adds quite of bit of energy to the substrate molecules involved. This greatly reduces the energy needed for the reaction to get started! Once initiated, the reaction will continue in the same way it would have without the enzyme. This means that the final products of a reaction catalyzed by enzymes have the same overall change in energy as if the reaction had taken place without an enzyme.
This catalytic property is essentially a way that cells can utilize different molecules at a much faster rate than basic chemistry would allow. In other words, the catalytic property is why your body can access the energy found in glucose in a matter of minutes – instead of the thousands of years it would take for glucose to naturally break down. We’ll cover this topic in more detail in section 3.2.
Enzymes also have the property of specificity. In other words, enzymes are very specific to the substrates they are meant to act on. If the wrong molecule tries to enter the active site, the molecule will be rejected. This means that an enzyme can only function on one, very specific type of substrate molecule.
Furthermore, enzyme specificity can target many different aspects of a substrate molecule. For example, the active site may have a specific charge that can only bind with substrate molecules that have opposite specific charges. Enzymes are typically extremely specific, even selecting against isomers and enantiomers of the substrate they are supposed to work on.
The specificity of enzymes ensures that only a specific substrate can form the enzyme-substrate-complex structure, induce a conformational change in an enzyme, and receive the energy needed to catalyze a specific reaction. This helps cells ensure that their enzymes are not randomly conducting reactions throughout the cell.
One of the most important properties of all enzymes is that the catalytic reactions they engage in are reversible. In other words, the same enzyme is often responsible for breaking two substances apart and putting them back together, or vice-versa.
For instance, consider a certain catalytic protein normally breaks a substrate down into smaller monomers. Under typical conditions, this enzyme might only work in one direction. However, if the products are building up on one side of the reaction, the enzyme may reverse its function. In this case, the enzyme would be combining two substrate molecules into a single product molecule.
This is important for a number of enzymes in your body that deal with energy production. For example, the enzyme “creatine kinase” is present in muscle and nerve cells and allows cells to store large amounts of energy that can be quickly accessed. When the cell has built up a large store of ATP molecules, creatine kinase transfers some of the phosphate groups onto creatine molecules, creating phosphocreatine and ADP molecules. This reduces the amount of ATP in the cell, allowing the cell to create more. If the muscle cell needs to use a ton of ATP very quickly, creatine kinase simply reverses and converts the phosphocreatine and ADP back into ATP for the cell to use!
The last property of enzymes that is important to cells is that enzymes are temperature and pH specific. This means that they can only function when the temperature and acidity of the solution are just right. If an enzyme falls out of these temperature and pH ranges, it will become denatured. The amino acid sequence of an enzyme has evolved to operate in specific temperature and pH ranges, which is why some enzymes can survive high temperatures or low pH balances, while others cannot.
This is important to cells for two reasons. First, since enzymes are only functional at specific temperatures and pH values it means that cells can more effectively separate their catabolic and anabolic reactions through compartmentalization. Certain compartments (such as lysosomes) are kept very acidic and the enzymes in these compartments only work within this acidic environment. If the enzyme were to escape into the cytosol it would quickly denature before it could start breaking down the inside of the cell.
Second, it means that cells and organisms must expend energy to regulate their temperature and pH in order to keep their enzymes functional. This is why cells have a number of integral membrane proteins that are constantly regulating the flow of ions and substances into and out of different compartments. Plus, almost all organisms have behavioral and physiological mechanisms that help regulate their temperature in changing environments – such as sweating or finding a shady spot when the temperature gets too high!