Amphipathic Definition
An amphipathic molecule is a molecule that has both polar and non-polar parts. Phospholipids, for example, have non-polar fatty acid “tails” and polar phosphate “heads.”
“Polarity” is an important property of molecules that determines how they will interact with other molecules.
Polarity is created when some atomic nuclei in a molecule attract electrons more strongly than others. The result is that the negative charge of the electrons congregates more around one atom than another, while the other atom possesses a slight positive charge because the electrons are closer to the first atom.
Polar molecules often contain elements like oxygen and sulfur, whose nuclei attract electrons very strongly. This allows them to pull some electrons away from their partner atoms.
Water is a good example of a polar molecule – its oxygen atom pulls atoms away from its hydrogens.
Non-polar molecules, on the other hand, are often heavy on elements like carbon, which has a fairly average pull on electrons. This means that carbon molecules are likely to share electrons equally and have a neutral charge.
In the case of polar molecules, “like attracts like” – polar molecules tend to interact strongly with other polar molecules, because their positive and negative ends are attracted to each other.
Non-polar molecules, on the other hand, do not interact strongly with polar molecules and may actually be pushed out of the way by other polar molecules that are attracted to the polar molecules’ partial charges.
Amphipathic molecules are biologically useful because they can interact with both polar and non-polar substances.
This allows them to make things possible that would not be possible with polar and non-polar molecules alone, including the creation of such crucial structures as the cell membrane.
Function of Amphipathic Molecules
Probably the most important function of amphipathic molecules in biology is in the formation of the cell membrane.
For life as we know it to exist, it is crucial that the materials of life – such as DNA, proteins, and energy molecules – are contained within a membrane. This increases the chances of the molecules interacting, and protects them from environmental threats.
Can you imagine a cell existing if its DNA, proteins, and sugars were floating around at random in a lake? Some scientists think that life may have started this way, but it’s not very efficient! Among other things, without cell membranes it would be impossible for living things to develop big structures like the human body that could exist outside of water.
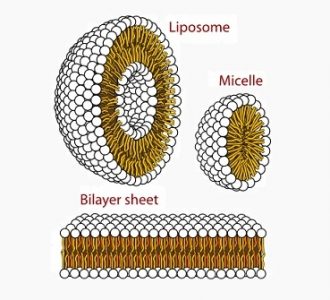
Amphipathic molecules accomplish this remarkable feat in a deceptively simple way. Phospholipids – the type of amphipathic molecule that makes up most cell membranes – are able to form a stable membrane because their “head” is attracted to water molecules, while their “tails” are repelled by them.
That means that phospholipids can form a stable membrane that is impermeable to most substances just by sticking together.
In most cell membranes, the non-polar “tails” of phospholipids congregate together inside of the membrane, while the polar “heads” stay on the outside, interacting with water inside and outside of the cell.
This configuration is stable because the polar heads “want” to interact with polar water molecules at all times, while the non-polar tails “prefer” to interact with other non-polar tails.
Having both polar and non-polar parts is also useful for some proteins, especially proteins that need to span both the polar and non-polar parts of the cell membrane to do their job.
Outside of cells, amphipathic molecules have another extremely useful function: most soaps and shampoos are made of amphipathic molecules!
Soaps work because their molecules combine polar sections, which will stick to water, with non-polar sections, which will stick to other non-polar molecules like grease, oil, and most other substances that won’t wash away with water alone.
Many substances, including grease, won’t wash away with water because they are non-polar. As such, grease molecules have no “desire” to interact with water molecules, so they just kind of sit there while you scrub them.
Adding soap, however, with its amphipathic molecules, gives grease molecules something that they “want” to interact with. Other parts of the soap molecules then stick to water, and the soap molecules take the grease with them when they wash away!
Examples of Amphipathic Molecules
Examples #1: Phospholipids
As described above, phospholipids are molecules whose amphipathic properties make life as we know it possible.
They are the most important component of cell membranes, and also form organelle membranes that allow cells to carry out their metabolic functions more efficiently.
Membranes made of phospholipids inside chloroplasts allow plant cells to harvest energy from sunlight in the process of photosynthesis, which is crucial to life on Earth. Phospholipid membranes in our own mitochondria allow our cells to liberate lots of energy from sugars through the process of aerobic respiration.
Other organelles that use phospholipid membranes to perform life functions more efficiently include the nucleus, the endoplasmic reticulum, the Golgi apparatus, and vesicles.
Examples #2: Soap
Amphipathic molecules allow detergents, soaps, shampoos, and many other cleaning products to carry away substances that don’t wash away with water alone.
Soaps are traditionally made by treating fatty substances, such as vegetable oils or animal fat, with a chemical called lye. Lye – an ionic compound like salt – creates a polar “head” on the fatty acid molecules, resulting in molecules that will both bind to grease and wash away with water.
Examples #3: Membrane Proteins
The most useful function of phospholipid membranes comes from their ability to separate two different chemical mixes. Cells take advantage of that property to create and use energy, including during photosynthesis, aerobic respiration, and the firing of neurons.
However, to create and regulate two different chemistries, cells must be able to selectively move substances back and forth across membranes. This creates the need for transport proteins that cross both the polar and non-polar portions of the cell membrane.
To be stable in their role as gatekeepers of the membrane, membrane proteins themselves must have regions that bond to both the non-polar interior of the membrane, and the polar outer layer.
Receptors – proteins that monitor one side of the membrane for chemical signals, and produce changes on the other side of the membrane if they receive a signal – are another common type of protein that needs to bond with both the polar and non-polar parts of the cell membrane.
Structural proteins that give a cell control over the shape of its membrane must also have this property.
In general, any protein in the cell that must work within the membrane needs to have both polar and non-polar regions.
Related Biology Terms
- Cell Membrane – The membrane that separates the inside of a cell from the outside of a cell.
- Lipid – A non-polar molecule consisting of many carbon and hydrogen atoms which share electrons equally.
- Polar – A term for molecules whose atoms share electrons un-equally, resulting in partial positive and negative charges throughout the molecule.
Quiz
1. Which part of a phospholipid is polar?
A. The fatty acid tail
B. The phosphate head
C. Both of the above
D. None of the above
2. Which organelles use phospholipid membranes to perform their functions more efficiently?
A. Chloroplasts
B. Mitochondria
C. Nucleus
D. Endoplasmic Reticulum
E. All of the above
3. Which of the following is most likely to be an amphipathic molecule?
A. A sugar
B. A DNA molecule
C. A membrane protein
D. None of the above