Ammonification Definition
Ammonification is part of the five-step nitrogen cycle, which is crucial for providing living organisms with the essential nitrogen that they need. Ammonification itself takes place thanks to the existence of decomposers, which break down animal and plant cells into simpler substances, making nutrients available in the ecosystem. The process of ammonification converts organic nitrogen, which is the way nitrogen is contained in compounds in living organisms, into inorganic ammonia (NH3) or ammonium ions (NH4+). Some examples of nitrogen-containing compounds in us and in other living organisms are proteins, nucleic acids like our DNA, vitamins like B-vitamins, and urea. The decomposers in this case are different bacteria and fungi that feed on these compounds, releasing ammonia, which in turn forms ammonium compounds in the soil to be taken up by plants. You can make yourself familiar with the nitrogen cycle by looking at the diagram below. Notice how the decomposers are shown feeding on plant and animal matter to produce ammonium.
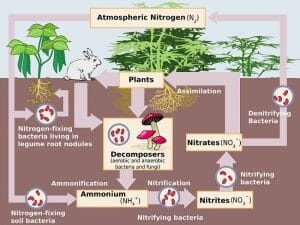
Function of Ammonification
Despite the atmosphere being composed of 78% nitrogen, living organisms can’t use the form of nitrogen contained in the atmosphere, which is N2, and that’s what makes the nitrogen cycle essential for life. When it comes to ammonification specifically, a dead animal or plant body and their wastes include organic nitrogen. This nitrogen has to return to the ecosystem in forms that can be used by living organisms; that’s where ammonification comes in to return nitrogen to soil or water in a way that allows plants to take it up and pass it along the food chain.
Can There Be Too Much Nitrogen?
Although nitrogen is used to make many compounds in living organisms, there comes a point at which the environment can contain far too much of it. Nowadays, people add fertilizers to soil to increase the amount of nitrogen present for ammonification, but this and other agricultural practices can lead to problems such as overgrowth of algae and other organisms in nearby bodies of water due to nutrient leaching, leading to toxicity and imbalances in ecosystems.
Related Biology Terms
- Nitrogen Fixation – Part of the nitrogen cycle; it’s where atmospheric nitrogen (N2) is converted into nitrogen compounds for plant and animal use.
- Denitrification – Part of the nitrogen cycle; it’s the reduction of nitrates in the soil to molecular nitrogen, which is then released into the atmosphere.
- Eutrophication – Pollution of a lake by sewage or fertilizer, which stimulates growth of algae and leads to outcomes that include depletion of oxygen in the lake.
Quiz
1. Which of the following is a product of ammonification?
A. Amino acids
B. Nitrites
C. Ammonia
D. Nitrogen
2. An example of ammonification is:
A. Converting nitrogen into ammonia by nitrogen fixers
B. Converting urea into ammonia by decomposers
C. Converting nitrates into ammonia by nitrogen fixers
D. Converting nitrates into ammonia by decomposers
