Definition
Oxygen saturation, measured with a pulse oximeter, is the amount of oxygen (in percent) bound to hemoglobin in red blood cells. The shortened form of the word is SpO2 – saturation of peripheral oxygen. This ‘fifth vital sign’ is an essential parameter for medical professionals in combination with body temperature, pulse rate, respiration rate, and blood. SpO2 results indicate how well oxygen is distributed in the blood.
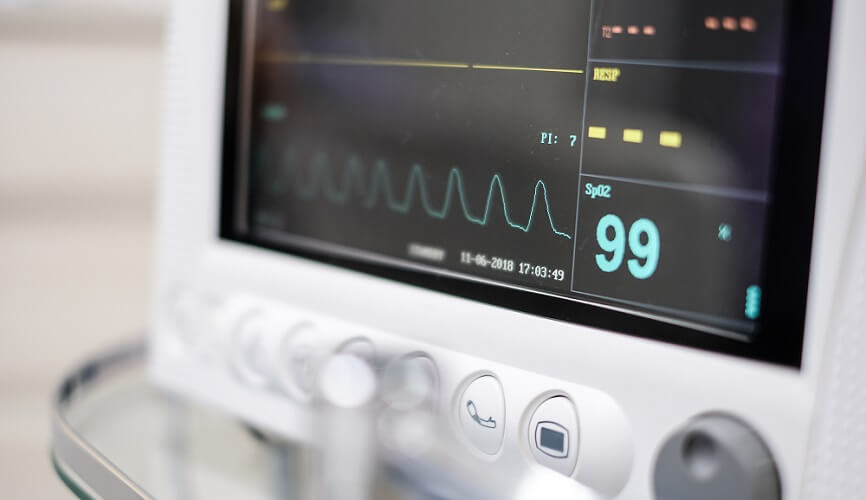
Blood Oxygen Saturation
Blood oxygen saturation results show a medical professional whether the blood transports oxygen efficiently. As the heart and brain are particularly vulnerable to low oxygen levels, it is important to know that sufficient oxygen is available.
Blood oxygen saturation measures certain aspects of oxygen supply but cannot tell us if all organs are receiving it. A blocked blood vessel or the pressure of a tumor can lower oxygen levels in localized tissue without an observable difference in a pulse oximeter reading.
Non-invasive pulse oxygen saturation (fingertips and ear lobes) results are best interpreted by someone with broad medical knowledge.

Fairly recent technology has also given doctors devices for measuring regional oxygen saturation (rSO2). It is now possible to measure oxygen saturation in the brain (cerebral oximetry) and inside the heart (intracardiac oximetry). Another way of measuring overall blood oxygen saturation is via an arterial blood gas (ABG) test.
Oxygen Supply
For someone to have an efficient oxygen supply, oxygen must reach the functioning part of the lungs. Oxygen (O2) can cross from the lungs to the blood circulation from the alveoli (alveolar gas exchange). Blood must contain enough hemoglobin to bind with the oxygen. The heart will also need to be strong enough to pump blood around the body. If all of these criteria are met, normal blood oxygen saturation levels should be expected.

Our blood absorbs oxygen in three ways:
- In the blood plasma (unbound O2) – a small amount – around 2%.
- In the water content of red blood cells (erythrocytes) – a tiny percentage
- Bound (reversibly) to hemoglobin – approximately 98%
Hemoglobin
Hemoglobin (Hb) is a protein made of four polypeptide subunits. These are composed of a heme group and a globin chain – hence the name hemoglobin. How well we produce hemoglobin depends on our HBB gene. If you want to know how hemoglobin is produced, this page gives a detailed description.
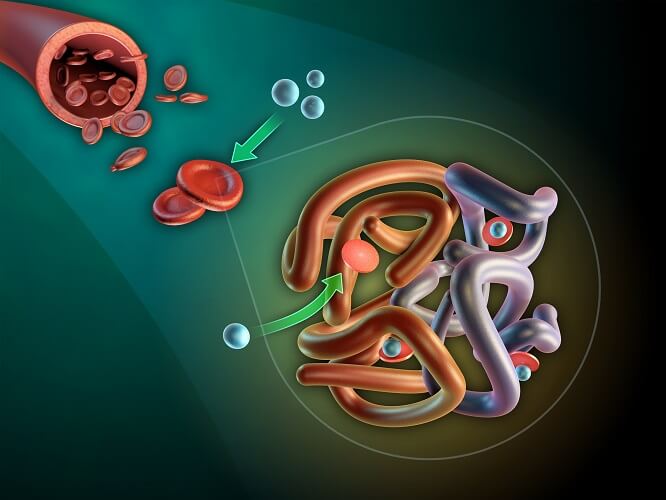
Each heme group surrounds an iron ion – it is the iron that binds to O2. This is why people with anemia are advised to take iron supplements.
When a hemoglobin molecule has bound to four oxygen molecules, it is referred to as saturated. It also turns bright red. Arterial blood that brings oxygenated blood from the lungs to the heart is, therefore, a brighter red than venous blood.
One gram of hemoglobin binds to 1.34 ml of oxygen in the average person. Hemoglobin concentration in the blood is (also on average) 15 grams per 100 milliliters. This means that each milliliter of blood has the potential to transport around 0.2 ml of hemoglobin-bound oxygen.
In reality, this is rarely the case. The conditions for perfect oxygen uptake depend on the oxygen-carrying capacity of hemoglobin.
Hb Oxygen-Carrying Capacity
Hemoglobin oxygen-carrying capacity is a self-explanatory term but not a simple topic. How oxygen binds to hemoglobin will not be discussed in detail in this article.
For good blood oxygen saturation levels, there must also be a good arterial oxygen supply. The pressure this gas produces within the confines of an artery is called the arterial partial pressure of oxygen (PaO2). The PaO2 must be high enough for O2 to bind to Hb.
With healthy lungs and sufficient oxygen in the environment, PaO2 results will be normal. Arterial partial pressure only measures oxygen dissolved in blood plasma, not within the red blood cells.
One red blood cell can contain up to 300 million hemoglobin molecules. Each hemoglobin molecule contains four iron ions and each iron ion can bind with one oxygen molecule (two oxygen atoms). A single red blood cell, therefore, has the potential to bind with one billion two hundred million oxygen molecules.
Again, this is never the case. About 10% of carbon dioxide in the blood binds (reversibly) to hemoglobin. Red blood cells also work less effectively as they age.
Other factors can negatively affect the oxygen-carrying capacity of hemoglobin:
- High levels of carbon dioxide
- High or low body temperature
- Increased blood acidity – a low blood pH is commonly the result of high CO2 levels
- Blood disorders that affect red blood cell production or function (like sickle cell anemia)
- Severe or chronic blood loss (less red blood cells resulting in less hemoglobin)
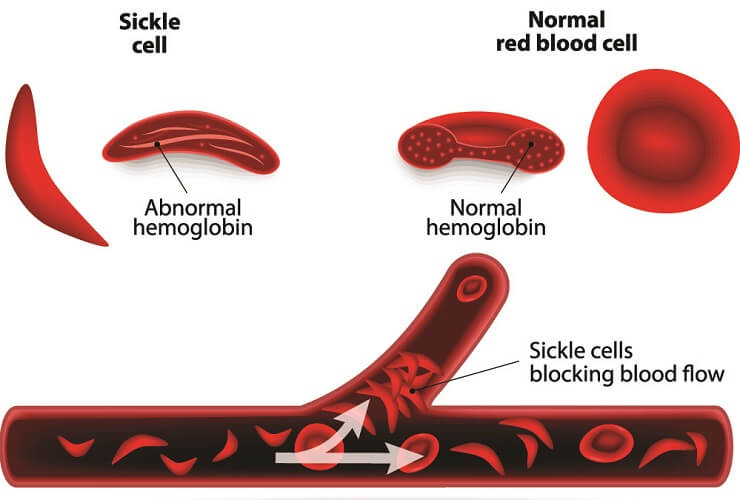
Hemoglobin can also bind to acidic hydrogen ions (H+) produced by the body to help stabilize the blood pH. If hydrogen ion levels increase (as with acute and chronic kidney insufficiency) this lower pH means hemoglobin molecules have a reduced oxygen-carrying capacity. Furthermore, H+ ions are more likely to bind to them and prevent some oxygen from binding to the iron ions.
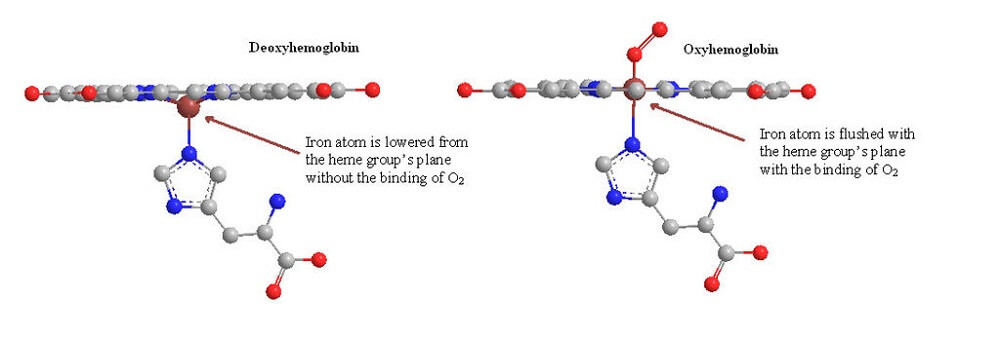
When bound to oxygen, Hb molecules are called oxyhemoglobin. When hemoglobin is not bound to oxygen is it called deoxyhemoglobin. In normal circumstances, oxyhemoglobin travels via the arteries to the tissues. After it has released the bound oxygen, it travels to the heart as deoxyhemoglobin. The heart pumps it to the lungs, and the process begins again.
How Do Oxygen Saturation Monitors Work?
An oxygen saturation monitor is most commonly found as a fingertip model. Probes can also be made to fit on an ear lobe. It is even possible to measure brain oxygen saturation with probes stuck to the forehead.
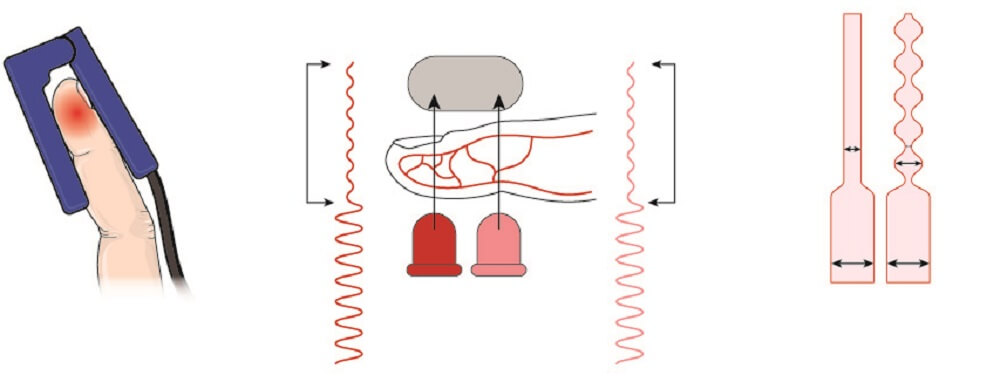
Fingertip oxygen saturation monitors work by passing light through the tissue of a finger. Light is emitted from one side of the probe and travels through the tissue to the other side. A calculation is made as to how much light has been absorbed. The higher the concentration of hemoglobin in the blood and the thicker the tissue, the more light is absorbed.
Oxyhemoglobin absorbs more light than unbound hemoglobin. This is also the case with hemoglobin bound to carbon monoxide (carboxyhemoglobin). A pulse oximeter cannot be used to diagnose carbon monoxide poisoning victims.
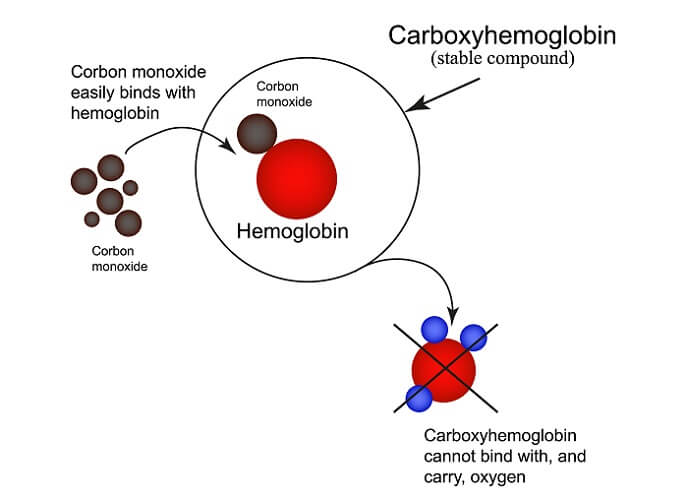
Two light sources are required to detect blood oxygen saturation levels:
- Red light (shorter wavelength)
- Infrared light (longer wavelength)
Oxyhemoglobin absorbs more infrared light; deoxyhemoglobin absorbs more red light than infrared.
An oxygen saturation device calculates the ratio of red and infrared light absorption. In a person with 100% SpO2, significantly more infrared light is absorbed than red light.
All oxygen saturation meters need to be calibrated before use as a medical device. This ensures the basic calculation is as accurate as possible. It is only calibrated according to the light absorption and O2 saturation ratio, not the distance between one side of the probe and another. A fat finger means the light must pass through more tissue. However, oximeters are called pulse oximeters for a reason.
These devices only look at light absorption in pulsating tissue – arteries. They calculate the amount of absorbed light in pulsating tissue. Using these results, they can also estimate tissue thickness and adjust the final measurement. Measuring pulsating tissue in people with low blood pressure or bad peripheral circulation gives inaccurate results. The probe cannot easily distinguish between low-pulsating and non-pulsating tissue.
This problem describes another feature of a pulse oximeter called the oxygen saturation perfusion index – a measurement of pulse strength. The strongest signal is 20%, the lowest is 0.02%. Current studies are looking at how the non-invasive (not inside the body) peripheral perfusion index can predict complications in broad groups of patients.
Oxygen Saturation Levels
Blood oxygen saturation levels show medical personnel the percentage of oxygen bound to hemoglobin. Anything above 95% is a normal SpO2 result.
Fingertip oxygen saturation readings can be unreliable when affected by multiple external (and internal) factors.
If an oximeter is placed on a cold finger with poor blood perfusion, results may be incorrectly low. An experienced nurse or doctor will realize the reading is faulty by looking at the patient’s skin tone. Low blood pressure also affects perfusion levels.
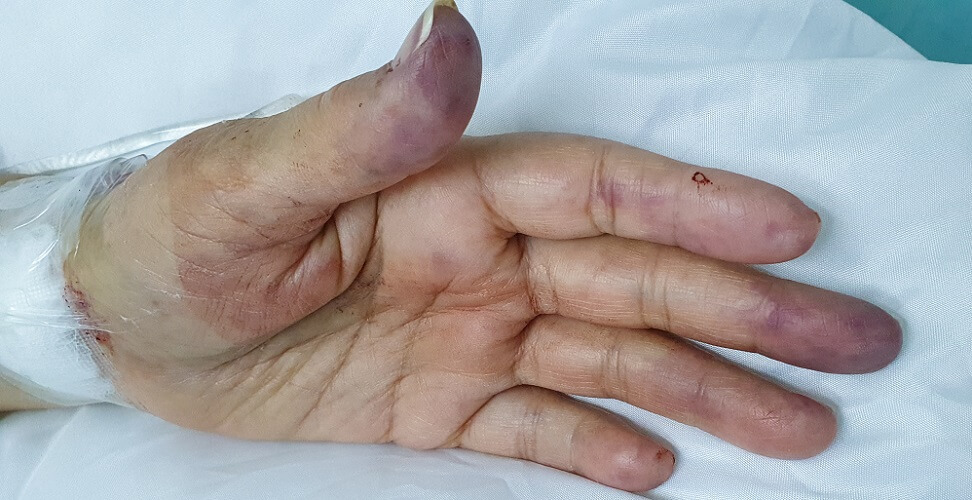
Gel nails do not seem to block the infrared signal of a pulse oximeter but older nail polishes do – the darker the color, the more disrupted the signal.
Respiratory disorders like chronic obstructive pulmonary disease (COPD) can produce permanent SpO2 levels of under 90%. In people presenting with the symptoms of covid, oxygen saturation should be kept above 90%.

It is impossible to have an oxygen saturation level of over 100%.
Normal Oxygen Saturation Levels
Normal range oxygen saturation is between 95% and 100%. The average SpO2 in healthy subjects is 98%.
The normal oxygen-carrying capacity (OCC) of one gram of hemoglobin is 1.34 ml. A healthy person has about 15 grams of hemoglobin in 100 milliliters of his or her blood. We can calculate how much oxygen is in 100 ml of this person’s blood if we measure the Sp02. If this is 98%:
15 g (Hb) x 0.98 (%) x 1.34 (OCC) = 19.7 ml O2/100 ml
Unfortunately, this result can be similar in a person with carbon monoxide poisoning or high H+ or CO2 levels. A pulse oximeter only measures bound hemoglobin (not bound oxygen) and does not make a distinction between gases.
Someone with chronic anemia will often have a normal oxygen saturation level. Even though they have fewer red blood cells, the hemoglobin in these few cells can bind to oxygen. Only with hemoglobin levels can we see (on paper) that something is wrong.
A low Hb of 7 g/dl shows that 98% is not a reliable parameter:
7 g (Hb) x 0.98 (%) x 1.34 (OCC) = 9.2 ml O2/dl
This result is less than half of that of the healthy subject.
<
<h3 title="Low SpO2“>Low Saturation Levels
Low saturation levels can have many interpretations:
- The sensor is damaged
- The person is cold
- The person is shaking or shivering
- The person is wearing nail polish
- A patient is hypoxic (low levels of oxygen)
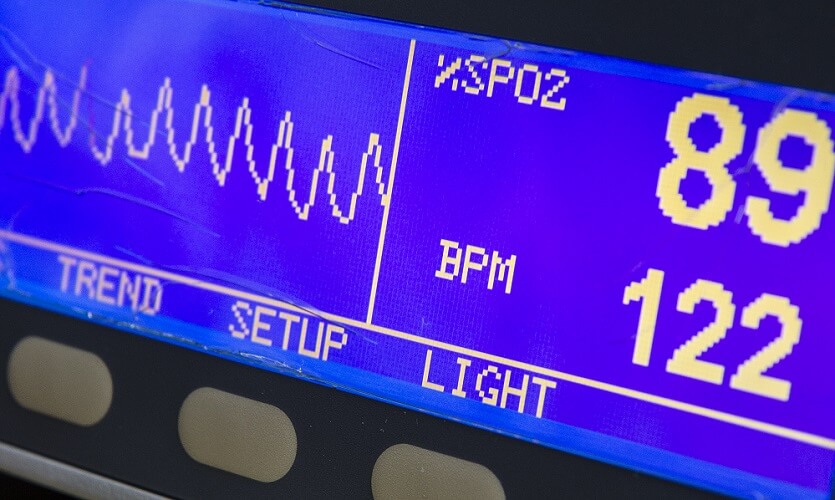
When the SpO2 reading starts to drop, this is a late response. It can take up to three minutes for a sensor to measure hypoxia. In young children, this time-scale is much less – sometimes just a few seconds.
This is because our blood carries more oxygen than our cells need (babies and young children use more oxygen in relation to their lung and circulatory capacity). Adult blood contains enough oxygen to supply a healthy person with oxygen for about three minutes.
A sudden drop in SpO2 levels indicates that the person has used up all available oxygen in the blood. This occurs if they are in an enclosed space without any oxygen (suffocation), have stopped breathing, or have lost a lot of blood. In cases of severe blood loss – even with a good oxygen supply – the heart cannot pump sufficient blood to the lungs.

Because of this, experienced paramedics and operating theater staff know that the pulse oximeter must be combined with extensive knowledge of the patient and additional measured data. This knowledge includes a patient history and the four vital signs of body temperature, pulse rate, respiration rate, and blood pressure.
