[LS1-6] Sugar to Carbon Molecules
This standard focuses on how and why sugar molecules contain the components needed to build many other kinds of organic molecules.
Resources for this Standard:
Here’s the Actual Standard:
Construct and revise an explanation based on evidence for how carbon, hydrogen, and oxygen from sugar molecules may combine with other elements to form amino acids and/or other large carbon-based molecules.
Standard Breakdown
If you look at this standard from an evolutionary perspective, it can help explain the origins of life.
Everything is Sugar
Carbon atoms, which can form 4 bonds with other atoms, create the backbone for all organic life. RNA and DNA have a sugar-phosphate backbone, essentially made of modified sugar molecules. Amino acids, though very complex, are also based on carbon-ring structures that were originally created from sugar and other molecules.
The endosymbiotic theory hypothesizes that eukaryotic cells formed out of symbiotic relationships between early forms of bacteria. This suggests that mitochondria and chloroplasts were originally free-living bacteria that got absorbed by larger cells. Going back even further, we can assume that these same biochemical pathways evolved over a long period of time. Thus, the origins of life were essentially self-replicating, sugar-based molecules, probably much like RNA.
Some Other Very Important Molecules
While the entire biochemical system that we know revolves around sugar molecules, here are some very important molecules that are synthesized from sugar:
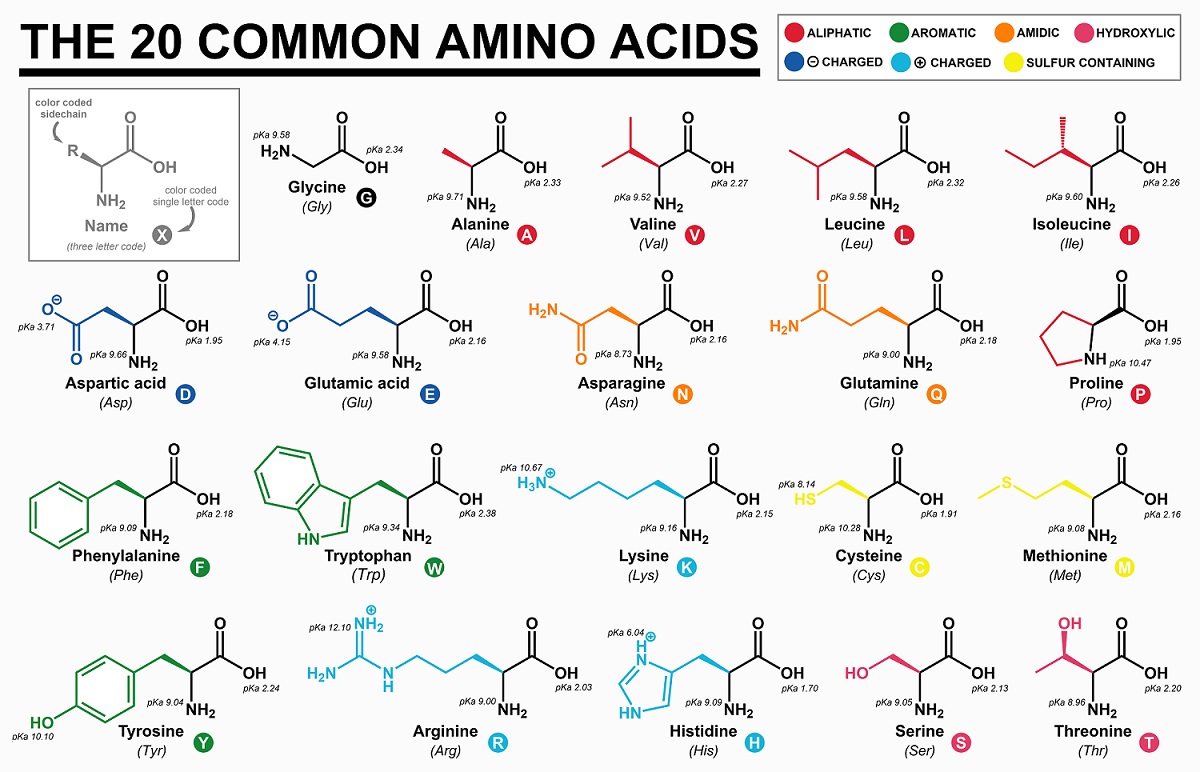
Amino Acids: These molecules are heavily modified sugar molecules, which can be linked together to form proteins. Though there are only around 21 different amino acids, they can be linked together in chains of thousands of amino acids to create a nearly infinite library of proteins – each which has a different function (or lack of function) in nature. The structure of each amino acid is very similar to glucose:
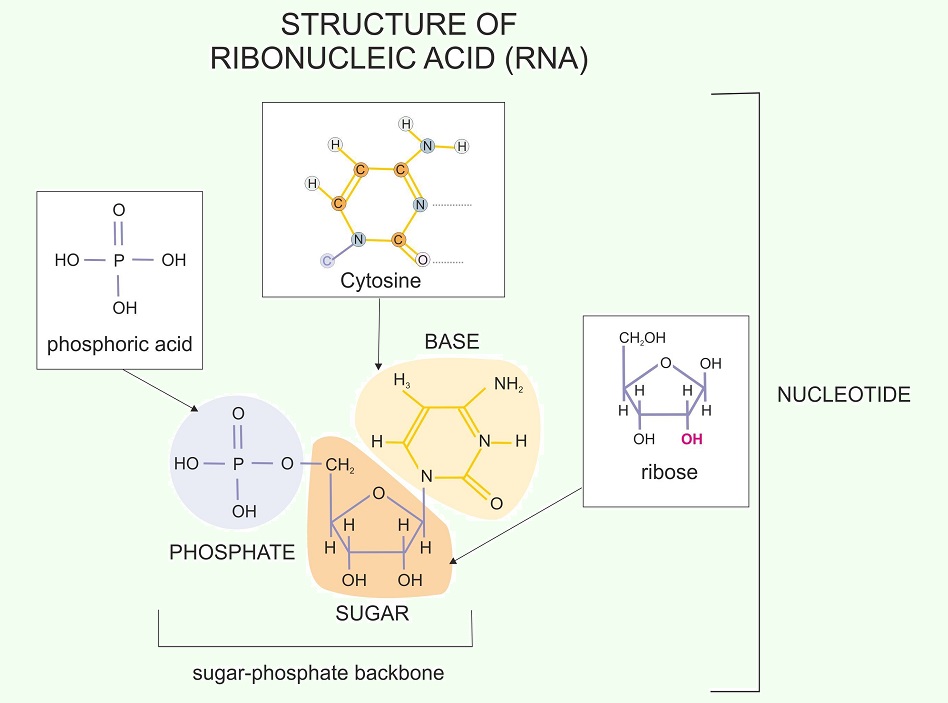
Nucleic Acids: Nucleic acids, the base molecules for RNA and DNA, are carbon-based molecules that can be synthesized using glucose as a starting point. In fact, the “backbone” of your DNA is made up of sugar-phosphate molecules, as seen in this image:
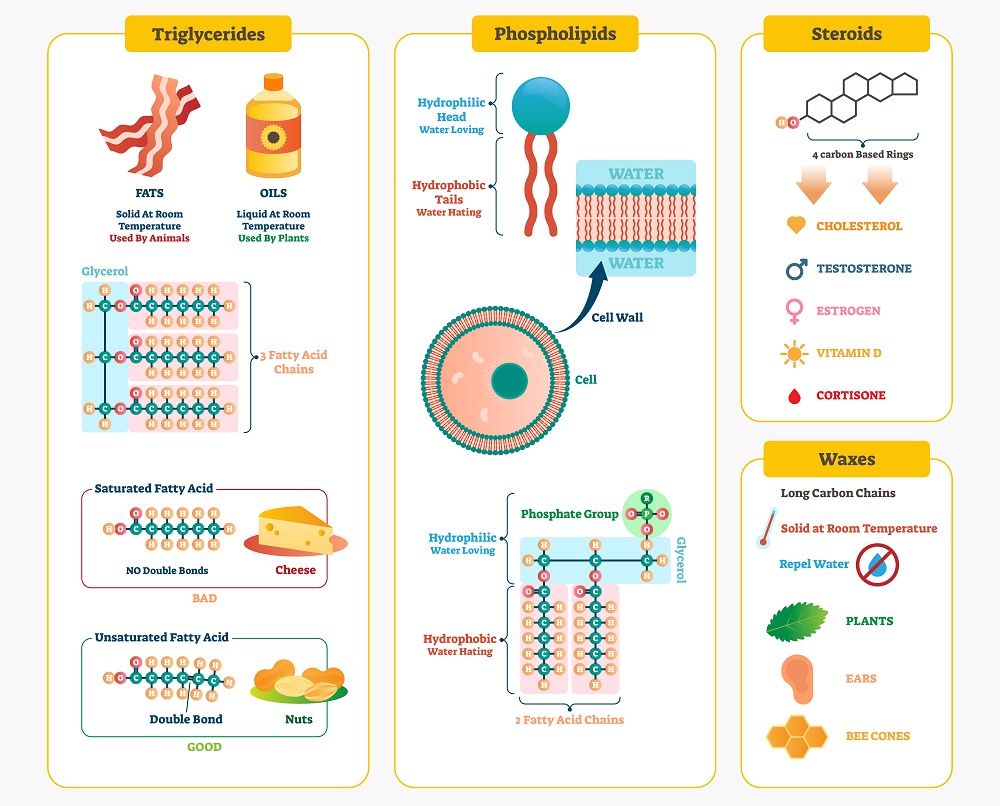
Lipids: Lipids, or “fats”, are simply long chains of sugar molecules that have been bound together for storage. Sugar molecules tend to take a lot of water within a cell, and for cells to store massive amounts of sugar they would likely explode with the amount of water needed. As an adaptation, cells have made many “storage” molecules from lipids. Likewise, the phospholipids that create the cell membrane are also essentially based on sugars, though unlike other lipids they have a water-loving phosphate group that keeps the lipid bilayer intact. You can see many kinds of lipids below:
Carbohydrates: Carbohydrates are essentially modified chains of glucose. While there are many carbohydrates in nature, the most common is cellulose. This molecule makes up the rigid structure of plant cell walls – allowing plants to stand tall with no bones or exoskeleton. Cellulose creation accounts for a huge majority of all sugar use, as cellulose is the most widely produced biological molecule on the planet! Cellulose is essentially a long chain of glucose molecules:

A little clarification:
The standard contains this clarification statement:
Emphasis is on using evidence from models and simulations to support explanations.
Let’s look at this clarification a little closer:
Models and Simulations
There are many “ball-and-stick” molecular representations on the internet that can help drive home the point that organic molecules are essentially modified glucose molecules. Instead of getting into specifics over how these are created, discussing the evolutionary model of why it is advantageous to base organic molecules on glucose and how evolution reinforces successful pathways can help explain why many molecules look and function like glucose.
What to Avoid
This NGSS standard also contains the following Assessment Boundary:
Assessment does not include the details of the specific chemical reactions or identification of macromolecules.
Here’s a little more specificity on what that means:
Specific Chemical Reactions:
This boundary is talking about how various biomolecules are synthesized. The process from sugar to amino acid, for example, contains dozens of highly technical intermediate reactions governed by the complex rules of organic chemistry and thermodynamics. Getting to this level of detail is unnecessary – it is sufficient if students grasp that all molecules are essentially rearrangements of glucose.
Identification of Macromolecules:
The images above should be used to explain and show how molecules have a common, sugar-based form. Having students identify these molecules on a test is unfair and is based in rote memorization, not a conceptual understanding of how molecules are related.
