Transferrin Definition
Transferrin is a crucial glycoprotein that shuttles iron in the blood. It would be an understatement to say that iron is vital for most life-sustaining processes. Transferrin has become an important biomarker for good health in the clinical setting, as it can reveal if a patient has functional iron depletion. This bio-marker, of course, will give a physician insight into a patient’s pathology, as well as which treatment plan will be most suitable moving forward.
Transferrin Structure
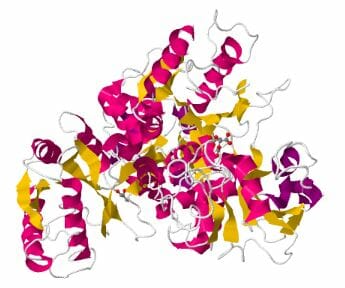
The image above is a 3-D depiction of human transferrin protein.
Structurally speaking, Transferrin is a polypeptide chain consisting of two carbohydrate chains and almost seven hundred amino acids. Transferrin has two homologous globular lobes, the N- and C- terminals comprised of alpha helices and beta sheets, with an iron binding site in between. The site itself is a six iron coordinate site occupied by a carbonate anion and four residues.
Each lobe is further divided into two clefts, or domains. Importantly, this structure lends transferrin the ability to undergo large conformational changes upon needing iron to be taken up or released. This is made possible by the rotating domains that rotate around a screw axis. Through x-ray crystallography, scientists have uncovered the mechanism for iron-release. This lies in how two of the basic residues from two of the domains will create a special hydrogen bond under neutral pH; however, this bond will break and thus release iron in the acidic pH of the endosome at its delivery site. Each transferrin molecule is able to carry two iron molecules in the bloodstream, and we will discuss in more detail the importance of sheltering iron until it is needed.
Transferrin Function
Iron is found everywhere on earth, and so it is no surprise that it also vital to sustaining life. Humans use iron for many cellular processes but perhaps the most important is iron’s ability to bind oxygen. As we know, oxygen is fundamental to cellular respiration and it is therefore necessary to transport oxygen from our lungs to each individual aerobic cell – without letting radical oxygen roam freely and ravage our cell’s membranes! Safe shuttling through our circulatory system is the answer. While humans contain about 3.7 grams of iron in our bodies, much of which comes from our diets, 2.5 grams will be “locked” inside hemoglobin with iron. Hemoglobin can then assume its role in transporting oxygen through the blood. However, just as importantly, we have evolved a way of recycling and storing this iron for future use. This is where transferrin comes in.
Plasma transferrin is a crucial player in iron metabolism. Transferrin essentially limits the levels of free iron in the blood. Free iron is dangerous in that it carries the risk of triggering free radical reactions, which sets off lipid oxidation and the destruction of thousands of molecules. Free radicals are defined as having at least one unpaired electron and they will thus be driven to steal electrons from every cell tissue including the heart, pancreas, brain, etc. Iron-triggered free radical damage can thus contribute to heart and liver disease, neurological issues, and more. Thankfully, transferrin binds essentially all circulating plasma iron. This chelation makes iron soluble and non-toxic as it is being delivered to tissues, accordingly serving the functions of rendering iron soluble, preventing iron-triggered free radical damage, and transporting iron. Transferrin, in fact, is the most valuable source of iron for red blood cells, with the highest turnover. The transferrin that circulates the blood is made and secreted by the liver. As previously mentioned, Transferrin can bind two iron ions. This is accomplished thanks its built-in iron (Fe3+) binding sites which have an extremely high affinity for iron. Lending to this affinity is an anion cofactor (preferably carbonate anion), that in its absence will make iron and transferrin binding negligible. The remaining four coordination sites are those from the transferrin molecule including an aspartic carboxylate oxygen, two tyrosine phenolate oxygens, and a histidine nitrogen. At any given time, about one third of the transferrin’s binding sites are filled. Upon radioactively labeling transferrin, it was found that about eighty percent of its iron was delivered to the bone marrow and then integrated into newly formed red blood cells. Other sites of delivery included the liver and spleen, which are major storage sites. It is said that of the 3 grams of iron found in adult human males, only about 0.1 percent of it ends up circulating in the plasma.
Clinical Significance of Transferrin
Tests measuring the levels of transferrin saturation are ordered when a healthcare provider suspects a patient has anemia. Symptoms may include pale coloration, fatigue, irritability, and shortness of breath. Anemia is defined as having low numbers of red blood cells, however one type is categorized by iron-deficiency. When iron levels run low in our bodies’ stores, our livers will upregulate transferrin synthesis in the healthy individual. Iron is necessary for hemoglobin synthesis, and thus having low levels of accessible iron will impede this process. Of course, there are multiple causes for anemia, which brings us to the Transferrin Saturation or Total Iron-Binding Capacity (TIBC) blood test. This test will determine if the underlying problem lies at the level of transferrin. This test checks how many of the possible transferrin binding sites end up “saturated,” or filled. In healthy individuals, transferrin levels range between 170 to 370 mg/dl and the percent saturated should lie between twenty and fifty percent. However, in severe iron-deficient cases this percentage may fall to under ten percent. Transferrin-iron saturation percentage will be low in patients with iron deficiency, and treatment options may include iron supplements or even blood transfusions.
Quiz
1. Which of the following best describes a main role of transferrin?
A. Systemic transportation of oxygen
B. Initiating radical pathways
C. Reducing levels of free iron
D. Preventing all anemia types
2. Which of the following was discussed as being necessary for transferrin-iron binding?
A. Oxygen
B. Carbonate
C. Calcium
D. Copper
References
- Mizutani, Kimihiko et al. “X-ray structures of transferrins and related proteins, Biochimica et Biophysica Acta (BBA) – General Subjects, Volume 1820, Issue 3, 2012, Pages 203-211, ISSN 0304-4165. https://doi.org/10.1016/j.bbagen.2011.08.003.
- Goodsell, David (2002). “Ferritin and Transferrin: molecule of the month.” PDB-101. Accessed 1 May 2018 from <http://pdb101.rcsb.org/motm/35>
- Harvard BWH (2001). “Iron Transport and Cellular Uptake.” Accessed 1 May 2018 from <http://sickle.bwh.harvard.edu/iron_transport.html>
- Iron Disorders Institute (2009). “How Iron Triggers Free Radical Activity.” Last accessed 2 May 2018 from <http://www.irondisorders.org/iron-tiggers-free-radical-activity>
- University of Rochester Medical Center (2018). “Transferrin.” URMC Health Encyclopedia. Last accessed 2 May 2018 from <https://www.urmc.rochester.edu/encyclopedia/content.aspx?contenttypeid=167&contentid=transferrin>
