Definition
Sulfuric acid (sulphuric acid) is a corrosive mineral acid with an oily, glassy appearance that gave it its earlier name of oil of vitriol. Other names are sulphine acid, battery acid, and hydrogen sulfate. The sulfuric acid formula, H2SO4, indicates the presence of a sulfur atom surrounded by two hydroxide compounds and two oxygen atoms. This powerful acid is used in various industries, primarily in fertilizer and chemical production.
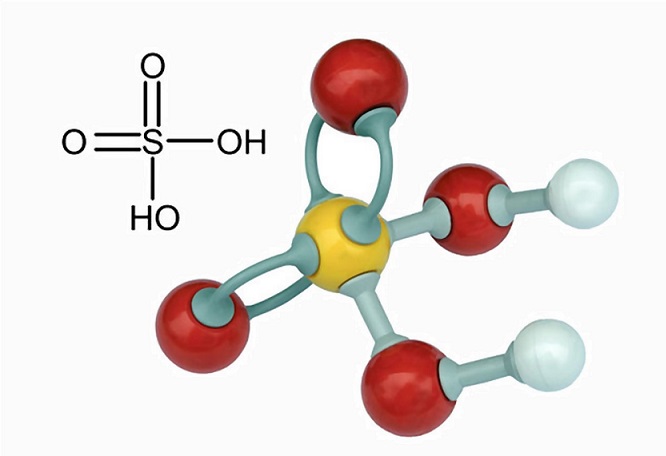
Sulfuric Acid Uses
Sulfuric acid uses are common in the industrial sector. This multifaceted acid is produced in large quantities and is the third most widely manufactured industrial chemical. First supplied on a large commercial scale in England in around 1740 through the burning of sulfur with potassium nitrate or saltpeter, today’s sulfuric acid is made using the contact process. Simple contact-processing plants burn molten sulfur to form the gas sulfur dioxide (SO2). This gas is cooled and then oxidized to form sulfur trioxide (SO3) at moderately high temperatures. Sulfur trioxide reacts with the hydrogen and oxygen molecules in water to form sulfuric acid. Sulfur trioxide is also called anhydrous sulfuric acid (without water), sulfuric oxide or sulfuric anhydride.

The other method of contact processing produces sulfur dioxide from materials that contain sulfur such as pyrite (iron (II) disulfide). This means the initial results can be extremely impure and it is important to cool off the SO3 gas to remove the majority of these impurities. Cooling also condenses water vapor which is siphoned off, as otherwise the next stage would lead to the production of vast quantities of toxic fumes. The remaining sulfur trioxide is dissolved or dried in concentrated sulfuric acid to produce H2S2O7 – a process known as fuming. Adding water to this ‘fuming sulfuric acid’ will create double the quantity of sulfuric acid – H2S2O7 + H20 = 2H2SO4.
In short, sulfuric acid production is split into five stages – the extraction of sulfur either from the ground or as a byproduct of other manufacturing processes, the conversion of sulfur to sulfur dioxide, a further conversion from sulfur dioxide to sulfur trioxide, and the final addition of water to turn sulfur trioxide into fuming sulfuric acid, and the addition of concentrated sulfuric acid to produce even more molecules.
Sulfuric Acid in Fertilizer Production
Fertilizer production uses sulfuric acid to add sulfur to the soil. Most agricultural land requires a source of sulfur to replace that which has been used by crops or leached during rainy periods. Sulfur deficiencies lead to leaf yellowing, leaf and tissue necrosis and stunted development. While plants cannot make use of elementary sulfur, soil bacteria oxidize it to form sulfate. Sulfate is the most important source of nutrition for all plant life and plants can easily absorb it through their roots.
During fossil fuel processing sulfur is extracted as a byproduct from the coal, crude oil, and natural gas that contain small to large quantities of it. During the refining of fossil fuels this sulfur is removed and most often sent in the form of sulfuric acid to fertilizer manufacturing plants. Not only is sulfur added to the soil, it is also necessary for superphosphate of lime production where rock phosphate is mixed with sulfuric and phosphoric acid. Superphosphate of lime enables plants to absorb phosphate. Another important fertilizer is ammonium sulfate, produced through the reaction between ammonium and sulfuric acid. Sulfuric acid produced for the fertilizer industry is technical grade or impure and slightly colored with a concentration of between 78 – 93%.
Sulfuric Acid in Chemical Production
Sulfuric acid uses in chemical production include caprolactam manufacture for nylon fibers and titanium dioxide which is a bright white pigment. In addition, sulfuric acid is necessary to produce the hydrofluoric acid that has replaced chlorofluorocarbons (CFCs) for use in refrigerators or air-co systems.
Sulfate salts such as calcium sulfate (plaster and gypsum) and hydrogen sulfate are derivatives of this particular acid. Although metal sulfites generally do not easily dissolve in water, other sulfate salts are quite the opposite, making sulfuric acid one of the most readily available and best solvents for use in a wide range of industries. Mineral supplements in the health sector and sodium laureth sulfate in shampoo and toiletries are other examples of sulfate salts. It is these particles that contribute to acid rain but at the same time, they may have a minor protective role as airborne particles towards the diffusion of solar radiation.
Sulfuric acid is also used to make explosives. Adding nitric acid and sulfuric acid to cellulose makes highly flammable nitrocellulose. For this reason, purchasing sulfuric acid in large quantities requires a license. Commercial grade sulfuric acid is sold at a minimum of 95% concentration.
Sulfuric Acid in the Laboratory
In the laboratory, sulfuric acid is used as a drying agent and in quantitative analysis where the concentration of a solution is calculated using a method known as titration. Whenever water is added to acid (beware the reaction with sulfuric acid and always add the acid to the water, not the other way round), positively charged hydrogen ions are released from the acid by way of proton donation. Bases or alkalis such as sodium hydroxide produce negatively charged hydroxide ions in the presence of water due to proton acceptance. Depending on the amount of acid or alkali in a solution, it is possible to calculate its concentration by adding its opposite and finding out when the negative and positive charges neutralize each other and join to form a water molecule. For example, when sulfuric acid and sodium hydroxide (NaOH) are mixed they will react and form water molecules. However, a metal such as sodium in the presence of sulfur and water will additionally produce sodium sulfate (Na2SO4) and water.
An example of titration use might be to determine the concentration of a sulfuric acid solution. Titration of acid requires a known concentration of an alkali reagent (titrant) – in this case sodium hydroxide. By adding small known quantities of the base to the acid and testing the neutralization reaction by way of a pH indicator, it is possible to work out how many moles of NaOH are required to react with H2SO4 and so calculate the concentration in moles per liter.
Sulfuric Acid in the Motor Industry
Sulfuric acid is an electrolyte; an electrolyte is a solution where ions are present. Lead-acid batteries in cars are composed of sets of positively-charged lead oxide plates immersed in an electrolyte, and negatively-charged pure lead plates, similarly immersed. This electrolyte is diluted sulfuric acid (approximately 33%). This is why sulfuric acid is often referred to as battery acid.
Car batteries store chemical energy and convert this into electrical energy through the reactions of hydrogen, oxygen, lead, and sulfur with each other. The presence of distilled (pure) water in sulfuric acid produces hydrogen and sulfate. Released negative electrons travel from the negative to the positive plate, while sulfate ions replace this loss in the negatively-charged plate (electrode), subsequently reacting with the lead to form lead sulfate. Sulfuric acid is perfect for this reaction as it is a diprotic acid able to release two of its protons at once.
This reaction also occurs in the positive plate where lead oxide bonds are broken and oxygen atoms dissolve into the sulfuric acid solution, leaving the lead atoms in the plate to bond with the sulfate. The added presence of oxygen and hydrogen in the solution produces water, lowering the sulfuric acid concentration. When this concentration is too low the battery needs to be replaced or recharged.
Recharging is the opposite of the above-described process, returning the battery to its original state of lead sulfate positive plate, pure lead negative plate and the original sulfuric acid electrolyte concentration; however, ions are slowly lost over time which is the reason why battery life, even when the battery is rechargeable, is limited.
On the subject of power, sulfuric acid is also a component of lithium-sulfuryl chloride (Li-SO2Cl2) and lithium-sulfur dioxide (Li-SO2) batteries. Lithium-sulfur batteries are receiving much interest at present as they, theoretically, can store significantly more energy than lithium-ion alternatives; however, much research still needs to be done.
Sulfuric Acid in the Food Industry
Sulfuric acid is often used in the food industry to dehydrate certain edibles like fruit or to stop the growth of bacteria and other harmful microbes that speed up the decay process. It is used in spray form to preserve meat or poultry. Sulfuric acid is also a general-purpose food additive used in alcoholic beverages and cheese.
For drying fruits, sulfuric acid’s ability to remove water is highly regarded. When in the presence of water, sulfuric acid reacts to form H3O+ (hydronium) and HSO4– (hydrogen sulfate). A subsequent reaction combines hydrogen sulfate with water to produce more H3O+ and SO42- or sulfate. As the hydration reaction of sulfuric acid does not require energy – it is thermodynamically favorable – and as it has many hydrogen atoms (protons) that it is happy to donate, sulfuric acid quickly lowers the pH of any tissue it comes into contact with it. However, in fruit drying processes concentrated sulfuric acid is not in direct contact with the fruit but instead used to dry the air around it, removing moisture and so aiding and accelerating the dehydration process.
Sulfuric Acid in the Human Body
In the human body, only the amino acids cysteine (nonessential) and methionine (essential) contain sulfur. This means that many proteins containing them are able to produce small quantities of sulfuric acid when metabolized. When this nonvolatile (metabolic) sulfuric acid is not broken down and used it is excreted via the kidneys.
Sulfur is one of the most abundant minerals in the human body and easily available in food sources from garlic to meat. Most hair and skin treatments, dietary supplements and pharmaceutical joint treatments contain plentiful quantities of this element.

The human body contains large quantities of free protons (H+) that help to maintain the body’s pH balance and enable a myriad of chemical reactions. The metabolism of proteins that contain cysteine or methionine provides a source of acidity that helps to maintain correct pH levels. In the bicarbonate buffer system, sulfuric acid combines with sodium bicarbonate to form sodium sulfate in the following reaction:
H2SO4 + 2NaHCO3 → Na2SO4 + 2H2O + CO2
The production of CO2 allows the body to rapidly regulate pH through pulmonary ventilation, while the kidneys regulate hydrogen and bicarbonate ions by way of excretion and reabsorption at a more leisurely pace.
Acid burns are the result of tissue dehydration. The corrosive characteristics of a strong sulfuric acid solution are due to its ability to donate hydrogen ions causing a powerful oxidation reaction. The skin contains large amounts of water and the interaction of H2SO4 with H20 produces positively charged hydronium ions (H3O+) and negatively charged hydrogen sulfate (HSO4–), the same reaction as described in the section describing the dehydration of fruit. As this reaction occurs it also generates heat which increases any damage.
The influx of sulfuric acid into the skin is partnered with the immediate dissociation of its hydrogen ions; the pH of the affected tissue drops rapidly. In this highly acidic environment, the surrounding cells die, leading to coagulation necrosis, eschars (dry, black tissue) and thrombus forming in the capillaries and larger blood vessels in the case of second and third-degree burns.
Adding water to a concentrated sulfuric acid burn or even a solution exceeding 20% not only adds an additional ingredient with which sulfuric acid can react but the subsequent reaction generates even more heat and so more damage. Rinsing under flowing water should only be done immediately after contact to flush the acid from the skin but is not a treatment or therapy. Before any treatment can be undertaken the acid needs to be neutralized. This is why it is sometimes advised to wash immediately with soap as most soaps are alkaline. With a sulfuric acid burn, time is of the essence. The old packaging information pictured below tells you to use a lot of soap then cover with magnesia or baking soda. All of these substances are alkaline. For sulfuric acid ingestion these instructions recommend drinking chalk, soap or even wall plaster, once again alkalis that will accept the dissociated protons. In both cases, the unlucky person is advised to get medical help.

Current MSDS information (material safety data sheet) tells us that sulfuric acid is extremely corrosive to all body tissues and only recommends flushing with warm water immediately after coming into contact with it. It is recommended to neutralize chemical spills on work surfaces and floors with alkalis but not human internal or external tissues. Medical attention should always be sought.
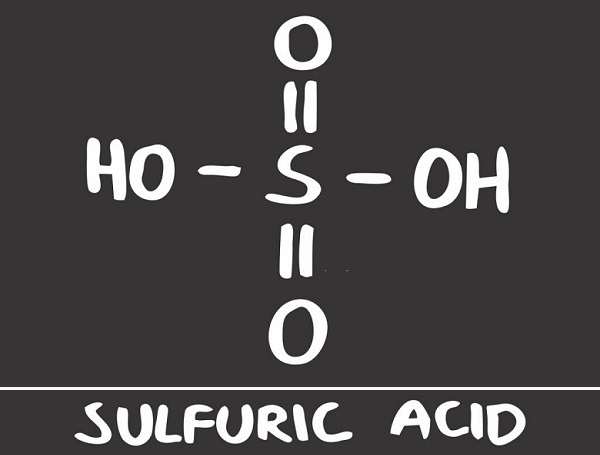
Sulfuric Acid Structure
Sulfuric acid structure is composed of a single sulfur atom bound to two oxygen atoms by way of double bonds, and two hydroxyl groups (OH) attached via single bonds. This is clearly seen in the image below.
When starting from scratch – from pure sulfur deposits – the first step towards producing sulfuric acid is the conversion of sulfur into sulfur dioxide during the burning of fossil fuels and also in the smelting process of impure aluminum, copper, zinc, lead and iron ores that contain sulfur. The structure of sulfur dioxide is shown below.

The next step is to produce sulfur trioxide from sulfur dioxide using heat in the presence of oxygen. Note that every one of the three oxygen atoms has a double bond. Sulfur trioxide is a liquid but reacts strongly with moisture in the surrounding air, as shown in this photo depicting a sulfur trioxide spill at the Marathon Oil research center in 1973.

The addition of water forms H2SO4 from SO3 and H2O. One molecule each of sulfur trioxide and water is necessary to make one molecule of sulfuric acid. The above vapor cloud is therefore composed of sulfuric acid.
Facts about Sulfuric Acid
Here are some sulfuric acid facts that will give you further insights into this corrosive, dangerous but important acid.
The molar mass of sulfuric acid is 98.08 g/mol. This is calculated according to the atomic masses of its atoms: two hydrogen atoms (2 x 1.008 g/mol), one sulfur atom (1 x 32.065 g/mol) and four oxygen atoms (4 x 16 g/mol). For the total weight of a single molecule of sulfuric acid, the calculation is the same but results are given in amu or atomic mass units. It is when you want to know the molecular weight of more than a single mole that the molecular weight result differs from the molar mass. For example, when you have 2 grams of sulfur dioxide (SO2 with a molar mass of 64.065 g/mol) and want to know how many moles this is. To calculate this result you first need to divide 1 (mole) with the molar mass of the substance. You then multiply the result by 2 (grams), as shown in the equation below.
Sulfuric acid density depends on its strength (concentration) and temperature. For example, a 13% H2SO4 solution at room temperature has a density of 9.09 g/cm3. A 96% solution at the same temperature has a density of 15.37 g/cm3. Sulfuric acid’s boiling point is 639 °F, 337 °C or 610 K; its freezing point is 37° F and its melting point is 50° F.
Sulfuric Acid and Autoprotolysis
Pure H2SO4 or anhydrous H2SO4 is a very polar liquid, meaning that it contains molecules where polar bonds – bonds between two atoms that are not equally distributed – have a very slightly negative and positive charge at opposing ends. These charged ends can attract or repel opposite-charged near-touching molecules by way of very weak dipole-dipole forces. Sulfuric acid completely ionizes in the presence of water into hydronium ions (H30+) and hydrogen sulfate ions (HSO4–). However, without water sulfuric acid will ionize with itself, where two sulfuric acid molecules autoprotolyze to produce one protonated sulfuric acid H3SO4+ ion and one hydrogen sulfate (HSO4–) ion as seen in the equation below:
2 H2SO4 ⇌ H3SO4+ + HSO4−
This high level of ionization makes sulfuric acid an excellent solvent for a wide range of reactions and well outperforms the solvent properties of water.
Sulfuric Acid in our Environment
Sulfuric acid is a constituent of acid rain and is formed by the atmospheric oxidation of sulfur dioxide gas in the presence of moisture. As sulfur dioxide is produced when fossil fuels are burned either by manufacturing plants, for electricity production and heating or by vehicles, acid rain is primarily a man-made phenomenon. Dry deposition in the form of acidic particles means acids can settle and later be washed away by rainfall, bringing acidic water into freshwater and marine ecosystems but also terrestrial environments. This can harm plant and animal life.
Sulfuric acid is also a product of the natural world through the oxidation of sulfur-containing minerals and ores. Volcanos are notorious for increasing the acidity levels of nearby lakes and rivers. It is said that the death of Pliny the Elder, who on an expedition to save friends from the Vesuvius eruption suddenly fell to the ground and died, was due to the inhalation of sulfur dioxide. Supposedly, once in the high moisture environment of the lungs this gas turned into sulfuric acid, overpowering and killing him.
The resulting acidic water flows caused by acid rain can lead to acid rock drainage (ARD). ARD is actually the result of diluted sulfuric acid formation through mineral reactions in the rock that is responsible for much environmental damage. Regulatory agencies try to limit the effects of ARD by keeping water away from industrial sulfur waste, adding alkalis to wastewater, and making sure wastewater is treated before it reaches natural water sources. Acidic water dissolves different metals present in sulfide ores and produces brightly colored but very toxic streams.
Quiz

