Rough Endoplasmic Reticulum Definition
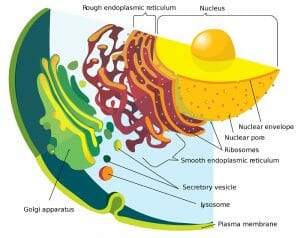
The rough endoplasmic reticulum (rough ER) is a part of the endomembrane system of the cell and a subset of the endoplasmic reticulum (ER). This organelle is primarily concerned with the synthesis, folding and modification of proteins, especially those that need to be delivered to different organelles within the cell, or secreted from the cell. The rough ER is also involved in the response of the cell to unfolded proteins and plays a role in the induction of apoptosis, due to its close interaction with mitochondria.
The rough ER is characterized by the presence of membrane-bound ribosomes that give it a distinctive appearance under the microscope. These ribosomes look like studs and distinguish the organelle from the smooth sections of the ER. Some proteins are also synthesized by strings of ribosomes, called polysomes. The rough ER can be identified by its morphology as well – it often consists of convoluted, flattened sac-like structures that originate near the nucleus. The lumen of the rough ER is contiguous with the perinuclear space and the membranes of the rough ER are associated with the outer nuclear membrane.
Structure of the Rough Endoplasmic Reticulum
The ER can be morphologically divided into two structures–cisternae and sheets. The rough endoplasmic reticulum is largely made of sheets – a two-dimensional array of flattened sacs that extend across the cytoplasm. In addition to ribosomes, these membranes contain an important protein complex called the translocon, which is necessary for protein translation within the rough ER.
The structure of the rough ER is also intimately involved with the presence of cytoskeletal elements – especially microtubules. When microtubule structure is temporarily disrupted, the ER network collapses and reforms only after the cytoskeleton is reestablished. Changes to the pattern of microtubule polymerization are also reflected in changes to ER morphology. Additionally, when ribosomes detach from sheets of rough endoplasmic reticulum, these structures can disperse and form tubular cisternae.
The edges of ER sheets have a high-curvature that needs to be stabilized. Proteins called reticulons and DP1/Yop1p play an important role in this stabilization. These proteins are integral membrane proteins that form oligomers to shape the lipid bilayer. In addition, they also use a structural motif that gets inserted into one leaflet of the membrane and increases its curvature. These two classes of proteins are redundant, since the overexpression of one protein appears to compensate for the lack of the other protein.
Functions of the Rough Endoplasmic Reticulum
The rough endoplasmic reticulum plays a number of roles within the cell, largely associated with protein synthesis. Polypeptides are synthesized, modified, folded into their correct 3-D shape and sorted towards an organelle or marked for secretion. It also plays an important role in modulating the response of cell to stress and in quality control for correct protein folding. When the number of unfolded proteins increases, cells alter their tubules:sheets ratio. This could arise from the greater area available within the sheets of the rough ER to rescue unfolded protein, or could reflect the need for the distinct proteome of the rough ER.
The rough ER’s proteome reflects its specific role within the cell. It contains enzymes involved in RNA metabolism that bind to and modify RNA. This is necessary since the organelle is involved in translating RNA into protein. It also contains proteins that recognize various signal sequences within a growing polypeptide, and aid in their translocation. Glycosylation enzymes and proteins that act as molecular chaperones that ensure proper folding of the synthesized polypeptides are also important proteins within this organelle. Occasionally, apoptosis is induced by the ER in response to an excess of unfolded protein within the cell. This function is mediated in consort with mitochondria.
Protein Synthesis
Translation for all proteins begins in the cytoplasm, after a processed mRNA transcript is exported from the nucleus. Translation begins with the binding of a ribosome to a mature mRNA transcript. However, after the first few amino acids are generated, some polypeptides are imported into the ER before translation can continue. This is based on the recognition of a short stretch of amino acids, also known as the signal sequence, by abundant cytosolic ribonucleoproteins called signal recognition particles (SRPs). SRP binding temporarily halts translation and allows the entire translation machinery to move towards the ER. At the ER, the nascent polypeptide is threaded into the organelle through transmembrane channels called translocons. These channels are made from a complex of proteins that allow the polypeptide to traverse the hydrophobic lipid bilayer of the ER membrane. The channel is not very wide, and therefore needs the polypeptide to be inserted as an unfolded string of amino acids. At this point, SRPs dissociate from the polypeptide and translation resumes. After the first few amino acids enter the lumen, ER resident enzymes often cleave the signal sequence. Newer amino acids are added to the growing polypeptide chain as the ribosome remains attached to the ER membrane, and the nascent protein continues to be inserted into the ER lumen. This process is called co-translational import into the ER.
The process of translation through membrane-bound ribosomes is particularly important for proteins that need to be secreted. Therefore, rough ER is prominent in liver cells that secrete serum albumin, cells of the digestive system that secrete enzymes, endocrine cells that synthesize and secrete protein hormones (such as insulin) and in cells that create the proteins of the extracellular matrix. Protein synthesis involving rough ER is also important for membrane-bound proteins, especially those like G-Protein-Coupled Receptors (GPCRs) that contain multiple hydrophobic stretches and traverse the membrane more than once through hairpin bends in their structure. The exact role of translocons and ER-resident proteins in completing the complex task of translating such proteins is not completely understood.
In the mammalian breast, the secretory system involving the rough ER is crucial during lactation. Single layers of cuboidal epithelial cells are involved in the main process of milk production. The nucleus in these cells is placed towards the basal end of the cell and the rough ER and Golgi apparatus are situated close to the nucleus. Proteins synthesized by the rough ER include the prominent milk protein casein, and whey proteins. These proteins are packaged into secretory vesicles or large micelles and travel through the Golgi network before fusing with the plasma membrane, releasing their contents into milk ducts.
Protein Folding and Quality Control
One of the side effects of being translated on the rough ER, with the polypeptide being translocated as an unfolded string of amino acids, is that these short stretches need to be protected until they can form their final 3-D structure, so that they do not prematurely form aggregates. One important mechanism to ensure correct protein folding is the glycosylation of the nascent polypeptide through enzymes called oligosaccharyltransferases. These enzymes are part of the translocon complex of the rough ER membrane. Glycosylation increases solubility of the peptide chains and protects them until molecular chaperons can bind to them and facilitate their folding. Prominent molecular chaperones of the rough ER include binding immunoglobulin protein (BiP), Calnexin (CNX) and Calreticulin (CRT). CNX/CRT assist in protein folding in consort with glycosylation. BiP contains a substrate-binding region that recognizes hydrophobic stretches in the polypeptide and an ATPase domain that powers its affinity for these stretches. Members of DnaJ/Hsp40 family of protein assist BiP in its task, modulating its ATPase activity, and enhancing its interaction with nucleotide exchange factors. The ER also contains enzymes that catalyze the formation of disulfide bonds and substrate-specific chaperones and enzymes that are necessary for certain proteins. It also maintains an oxidative environment to assist in this task.
BiP, CNX/CRT and other chaperones are enriched in regions of the ER that interact closely with mitochondria. This section of the ER is called MAM, or mitochondria-associated membrane. The MAM is emerging as an important signaling hub within the cell that integrates signals from the ER and plays a role in calcium homeostasis, autophagy, apoptosis and mitochondrial function.
In spite of these mechanisms to ensure that proteins are folded correctly, some need to be removed from the system, either due to errors in translation or due to genetic mutations leading to the production of defective proteins. This is accomplished by the quality control systems within the ER that ‘proof read’ newly synthesized proteins. When the polypeptide has not folded into its native state, molecular chaperones bind to the polypeptide again and make another attempt at folding the protein into its correct shape. When repeated attempts fail, misfolded proteins can be exported to the cytosol, and removed through the proteasome using ubiquitin-mediated protein degradation.
Protein Sorting
Once proteins are synthesized and folded, they need to be dispatched towards their ultimate destination. The first step in this process is the formation of vesicles from the edges of the rough ER. These vesicles carry cargo towards the Golgi network and are created by the coordinated action of a variety of proteins, starting from the vesicular coat protein complex II (COPII). A GTPase enzyme, and a nucleotide exchange factor are necessary for COPII to carry out its functions. Together, these proteins distort the membrane and allow the formation of a vesicle carrying appropriate cargo. Proteins that need to remain within the ER are moved back through retrograde transport from the Golgi using vesicles formed by a related protein called COPI.
Related Biology Terms
- Micelle – An aggregate of molecules containing both hydrophilic and hydrophobic regions dispersed in a liquid, forming a colloidal solution. In an aqueous medium, the micelles form with the hydrophilic regions facing water, and the hydrophobic regions sequestered towards the interior.
- Polysome – Association between a mature mRNA transcript and two or more ribosomes involved in translating the codons within the RNA.
- Proteome – Complete set of proteins expressed in a cell, tissue, organ or organism at a particular point in time.
- Ribonucleoprotein – Complex formed by the association of ribonucleic acid (RNA) with proteins.
Quiz
1. Which of these is true about the rough endoplasmic reticulum?
A. Crucial for synthesizing proteins that are secreted from the cell
B. Important during lactation and the production of milk
C. Studded with ribosomes and polysomes
D. All of the above
2. Which of these molecular mechanisms is directly involved in proper protein folding in the ER?
A. Binding of Signal Recognition Particles to a nascent polypeptide
B. Translocons on the ER membrane
C. Glycosylation and binding of molecular chaperones
D. All of the above
3. Which of these proteins is involved in anterograde transport from the rough ER to the Golgi apparatus?
A. Ubiquitin and the proteasome
B. CNR/CXT chaperone proteins
C. COPII
D. All of the above