The function of a protein is highly dependent on its 3D structure. The amino acid sequence of a polypeptide chain determines the final 3D structure of the protein.
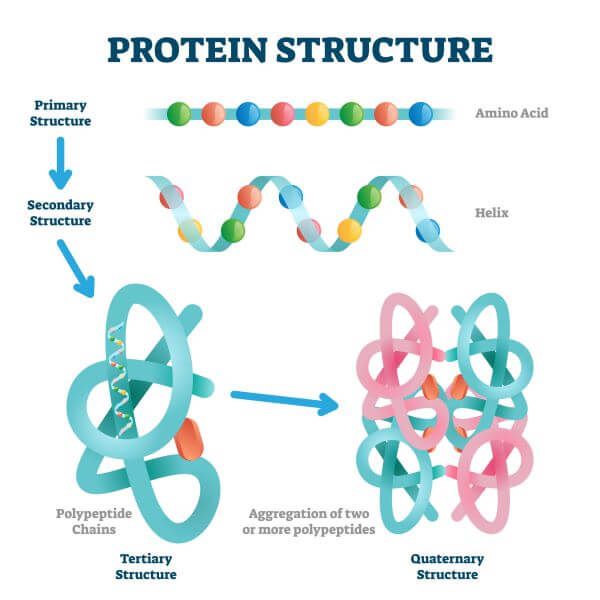
There are four levels of protein structure; the primary structure, the secondary structure, the tertiary structure, and the quaternary structure. Furthermore, there are two main classes of 3D protein structures; these are globular and fibrous proteins.
Complex, 3D proteins are produced by folding of the simple primary and secondary protein structures.
What Are Proteins Made Of?
Proteins are polymers, meaning they are large molecules made up of many smaller molecules. The small molecules that make up proteins are called amino acids.
Each amino acid contains a carbon atom, an amino group, a carboxyl group, and a side chain (also known as an R group).
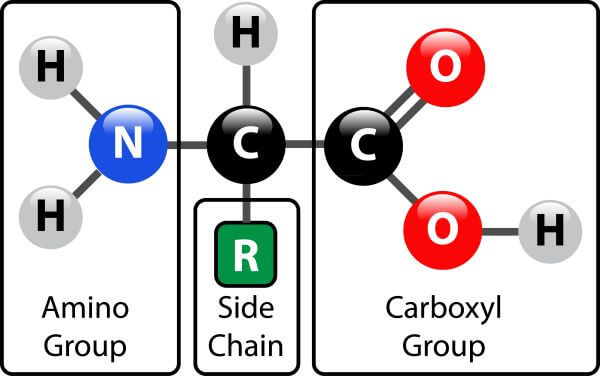
The side chain is the only variable component of the amino acid. The type of side chain identifies the type of amino acid and also determines its characteristics (e.g. its size, pH, and polarity). There are only about 20 different types of amino acid in the human body, but these can combine to make approximately 20,000 unique proteins.
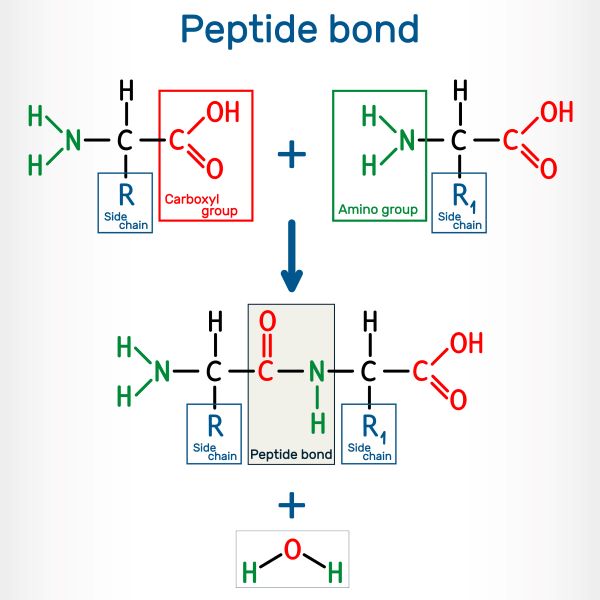
Amino acids are joined together by peptide bonds, which form between the amino group of one molecule and the carboxyl group of another. Two amino acids joined together is called a dipeptide; a chain made of multiple amino acids is known as a polypeptide.
Different Types of Protein Structure
The structure of proteins is directly related to their function and may be primary, secondary, tertiary, or quaternary.
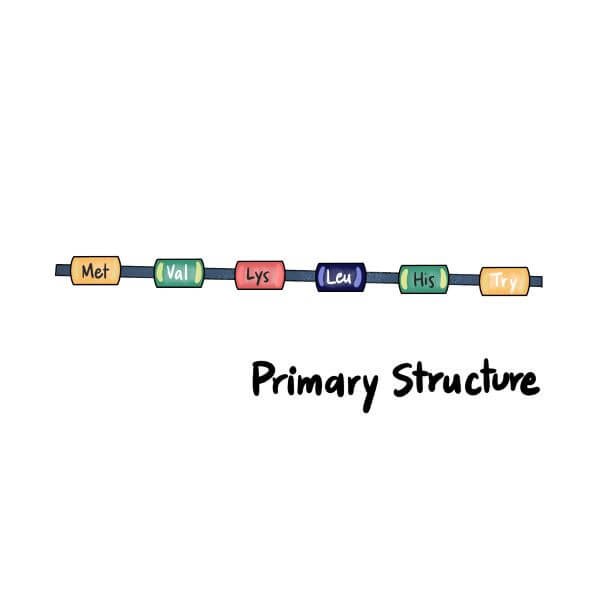
Primary Protein Structure
The most basic type of protein structure is called the primary structure. A primary protein is a simple, linear chain of amino acids (AKA a polypeptide chain).
The order of amino acids in the polypeptide chain is determined by the order of nucleotides (the DNA sequence) of the gene that encodes it. Even a tiny change in the amino acid sequence of the polypeptide chain can alter the overall structure and function of the protein.
Secondary Protein Structure
The secondary protein structure is made by folding of the polypeptide chain. The polypeptide chain folds up and hydrogen bonds form between the atoms of the polypeptide chain, holding the secondary structure in place.
There are two main types of secondary protein structures: the α-helix and the β-pleated sheet.

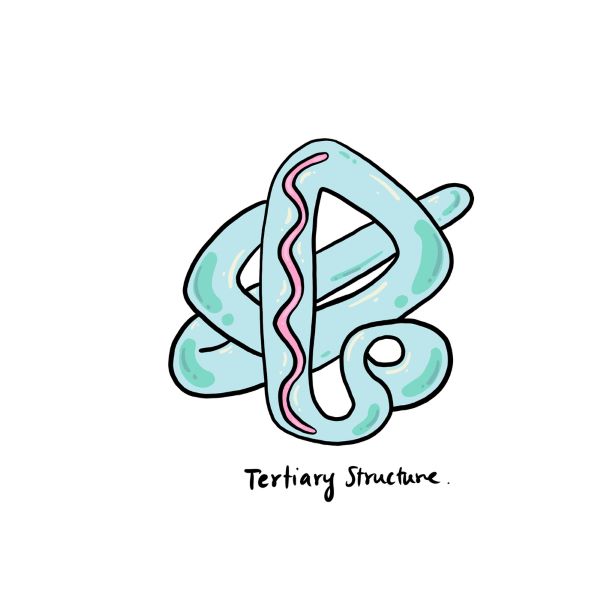
Tertiary Protein Structure
A protein isn’t fully functional until it has a 3D shape. The 3D structure of a protein is referred to as its tertiary structure and is made by further folding of secondary proteins.
Interactions between the side chains of amino acids lead to the formation of the tertiary structure, and bonds form between them as the protein folds. These include hydrogen bonds, ionic bonds, and disulfide bonds.
Disulfide bonds are covalent bonds that form between sulfur-containing side chains and are much stronger than other types of bonds. The disulfide bonds are what hold the tertiary structure of the protein in place.
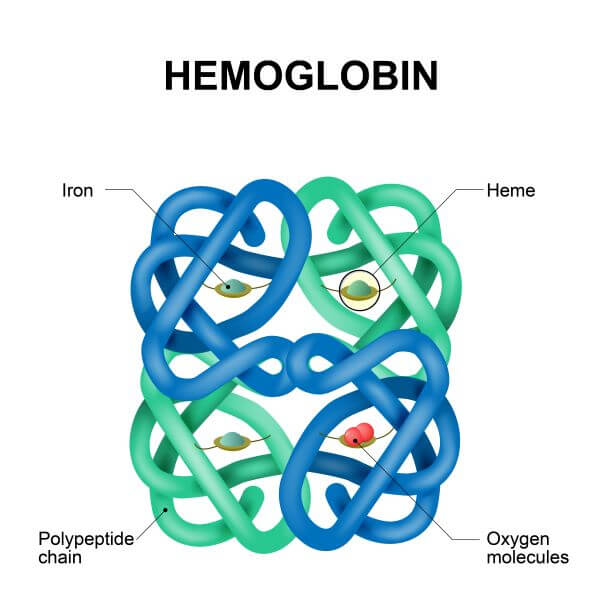
Quaternary Protein Structure
Many proteins are made of a single polypeptide chain and don’t become any more complex than their tertiary structure. However, some proteins are made up of multiple polypeptide chains. When several polypeptide chains (AKA subunits) come together, they can form a structure known as a quaternary protein.
One example of a quaternary protein structure is hemoglobin. Hemoglobin is made up of four polypeptide chains, and is specially adapted to bind oxygen in the blood.
Classes of Protein Structure
The function of a protein depends heavily on its final structure. Tertiary and quaternary proteins are both functional proteins with a 3D structure. However, the type of structure can vary significantly between different proteins.
There are two main classes of 3D protein structure: globular proteins and fibrous proteins.
Globular Proteins
Globular proteins are usually round and ball-shaped. They usually have metabolic functions, for example, they may be enzymes or antibodies. Haemoglobin is an example of a globular protein.
Fibrous Proteins
Fibrous proteins are long and narrow and usually have a structural function. Examples of fibrous proteins include collagen (found in bones, muscle, and skin) and keratin (the material that makes up hair, nails, and feathers).
What is Protein Denaturation?
Proteins are only functional so long as they keep their 3D structure. If they are unfolded and lose their shape, they will no longer be functional.
A protein will lose its 3D structure if the hydrogen bonds that hold it together are broken.
This may happen if the protein is put under stress, for example by heating, changes in pH, dehydration, or vigorous shaking. The protein may be able to return to its original shape once the denaturing agent is removed but, in some cases, denaturation is permanent. One example of irreversible denaturation is the protein in egg white, which becomes opaque as it loses its shape during cooking.