Porphyrin Definition
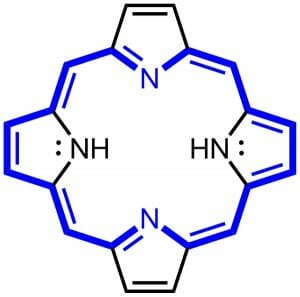
A porphyrin is a large ring molecule consisting of 4 pyrroles, which are smaller rings made from 4 carbons and 1 nitrogen. These pyrrole molecules are connected together through a series of single and double bonds which forms the molecule into a large ring. The technical name for 4 pyrroles connected together is a tetrapyrrole. The ring is flat in space, and the distribution of electrons is fairly equal around the circumference of the ring. For this reason, a porphyrin is considered an aromatic compound. This means that a porphyrin molecule is very stable. The model of a general porphyrin is called porphin. This molecule is only rarely found in nature as an intermediate, but it is the base of all porphyrin molecules. Porphin can be seen below.
The blue parts of the molecule represent the aromatic ring which forms the base of all porphyrin molecules. The black molecules and bonds will eventually be substituted for complex side chains. These molecules will allow the cellular machinery to attach to and use the porphyrin. Porphyrins are also capable of absorbing certain wavelengths of light, especially when associated with different ions. Porphyrins cause both the red color of blood and the green color of plants, as discussed below.
Porphyrin molecules serve a number of purposes in animals, plants, and even bacteria. For this reason porphyrin is considered an evolutionarily conserved molecule. This means that because of its usefulness, countless lines of organisms have used and modified porphyrins to fit their needs.
Types of Porphyrins
Porphyrins in Animals
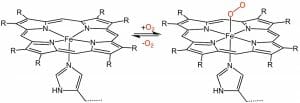
A major use of porphyrin molecules in animals is in the construction of heme groups. These molecules are simply a porphyrin molecule with various side-chains substituted around the main ring. In a heme, the porphyrin ring serves an important function. The nitrogen molecules at the center of the ring are capable of “hosting” an iron molecule. It is this porphyrin structure, holding iron, which gives blood its red color. While the nitrogen does not technically bind to the iron molecule, it is nonetheless held in place by the influence of the nitrogen molecules and their distribution in space. A general heme can be seen below.
The general purpose of a heme is to transport oxygen. This can be seen in the above image. When the oxygen is bound to the heme, it can be transported quickly around the body and through the cells. There is a specific protein associated with each part of the body which uses hemes to transport oxygen. The red blood cells have the protein hemoglobin, which holds the heme in place. These proteins also impart functionality to the heme. When hemoglobin is in an acidic environment, for instance, it changes shape. This change of shape forces the oxygen to detach from the heme. This mechanism has evolved because carbon dioxide makes the blood acidic, and is the by-product of oxygen usage. Therefore, when carbon dioxide is high, cells need more oxygen. This mechanism of hemoglobin allows the oxygen to be released in the correct parts of the body.
Another heme-holding molecule, myoglobin, functions as the oxygen transporting molecule within the muscle cells. This heme is also made from porphyrin, and hosts iron. Myoglobin has different side-chains than hemoglobin. As such, it can interact with the machinery of muscle cells, and deliver oxygen from the surface of the cell to the mitochondria which need the oxygen of oxidative phosphorylation. Heme groups, made of porphyrin molecules, are also seen in the cytochrome molecules of the electron transport chain. Here, the iron molecule functions like a depository for extra electrons. It takes on electrons when necessary, and releases them at other times. The electron transport chain is a complex series of cytochrome proteins, each hosing a heme group. These work in unison to transfer the energy out of electrons, driving a proton pump and storing energy in the form of ATP.
A porphyrin molecule is an organic molecule, and must be created and destroyed by specific proteins in the body. Because proteins are programmed by the DNA, any mutations in the DNA can cause malfunctions in the protein which process porphyrin molecules. While usually extremely helpful, a porphyrin which hasn’t formed properly or cannot be broken down poses a serious threat to the body. Porphyrin molecules are highly interactive. They can disrupt the cell membrane, and because they hold an iron molecule with the potential to act as an electron sink, they encourage the formation of free radicals.
The genetic condition which cause buildups of porphyrins due to their inability to be broken down or properly created is called Porphyria. There are many types of Porphyria, based on which enzyme becomes mutated. Because of this, there are a variety of symptoms and treatments for the different versions. Ultimately, the porphyrin molecule which is causing the issue must be identified and dealt with. This could mean substituting an artificial porphyrin, or putting the patient through gene therapy to get their body to produce the proper enzymes. Porphyria is often diagnosed by purple colored urine, caused by unbroken porphyrins left in the urine.
Porphyrins in Plants
Because the porphyrin molecules are evolutionarily conserved, we see the same iron-holding heme porphyrins in plants as we do in animals. Plants also use the electron transport chain to produce ATP, and similar porphyrins are used as those in animals.
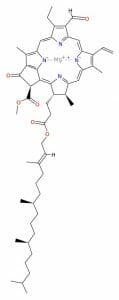
However, plants have also mastered a different configuration of porphyrin molecule, which allows them to capture the energy in sunlight. Chlorophyll is a special molecule designed around a porphyrin base. Seen below, the chlorophyll molecule has several unique side-chains off of the porphyrin molecule. It also has a really long side chain, seen coming off the bottom. These side-chains slightly change the shape and configuration of the base porphyrin.
You will notice that instead of iron (Fe), there is now a magnesium (Mg) ion “captured” within the porphyrin. Unlike heme groups, which contain iron, the magnesium in the chlorophyll is not capable of transferring oxygen. Instead, the molecule functions to capture energy from sunlight. The magnesium ion helps capture and store electrons during the process. The magnesium ion also helps the porphyrin absorb red and blue light, instead of reflecting red light as it does with blood. This causes only a strong green color to come through, which gives plants their green color.
Other Porphyrins
Beyond plants and animals, there are porphyrins almost everywhere in the world. Bacteria use porphyrins in a similar way to animal cells, although the final molecules they use may look very different from the hemes in animals. Certain bacteria also have the ability to photosynthesize, and like plants, they use porphyrins to capture the energy of the sun. There are also substances like vitamin B12, which are synthesize from a porphyrin to start, but hardly resemble one when complete.
Porphyrins can also be synthesized in the laboratory. These molecules, because they act as pigments, have been used in dyes and as color in various solutions. While we can synthesize many porphyrins in the lab, they tend to be more symmetrical than natural porphyrins. This is because our body uses enzymes developed over millennia to shape porphyrins into a useful shape. Our understanding of biochemistry is not yet advanced enough to replicate these processes in the lab.
Another interesting example of porphyrins can be found in crude oil. Apparently, during the many centuries buried under the Earth, organic molecules can naturally form into porphyrins. These substances are found as side-products when crude oil is extracted from the ground. Evolutionary scientists have hypothesized that these porphyrins, and those created in similar methods, could have been the starting material with which life on Earth first developed.
Quiz
1. There are currently 9 recognized types of Porphyria. Which of the following statements accurately describes the reason for this?
A. The genes encoding for porphyrin-changing enzymes can mutate in many ways
B. The different types of Porphyria simply represent different symptoms for the same disease
C. The 9 types are all caused by the same gene
2. Plants, animals, and bacteria all use a version of the Cytochrome protein, which houses a heme based around a porphyrin. What does this tell us about evolution?
A. Nothing
B. These organisms have a common ancestor
C. These organisms have no common ancestors
3. Porphin, the base molecule of a porphyrin, is not found in nature. Why is this?
A. Porphin can only be created in the laboratory
B. Porphin is an intermediate; cells immediately attach functional side-chains
C. Porphin is only created by extremely rare organisms
References
- Lodish, H., Berk, A., Kaiser, C. A., Krieger, M., Scott, M. P., Bretscher, A., . . . Matsudaira, P. (2008). Molecular Cell Biology (6th ed.). New York: W.H. Freeman and Company.
- McMahon, M. J., Kofranek, A. M., & Rubatzky, V. E. (2011). Plant Science: Growth, Development, and Utilization of Cultivated Plants (5th ed.). Boston: Prentince Hall.
- Nelson, D. L., & Cox, M. M. (2008). Principles of Biochemistry. New York: W.H. Freeman and Company.