Plasmolysis Definition
Plasmolysis is when plant cells lose water after being placed in a solution that has a higher concentration of solutes than the cell does. This is known as a hypertonic solution. Water flows out of the cells and into the surrounding fluid due to osmosis. This causes the protoplasm, all the material on the inside of the cell, to shrink away from the cell wall. Severe water loss that leads to the collapse of the cell wall can result in cell death. Since osmosis is a process that requires no energy on the part of the cell and cannot be controlled, cells cannot stop plasmolysis from taking place.
Plasmolysis and Osmosis
Osmosis is responsible for the occurrence of plasmolysis. Osmosis is a special type of diffusion that occurs when water flows into or out of a membrane such as a cell’s plasma membrane. It occurs based on the type of solution that a cell is in. A solution is a mixture that contains a fluid, or solvent (usually water), and a solute that is dissolved in the solvent. When a cell is placed into a hypertonic solution, there is a higher concentration of solutes outside the cell, so water flows out of the cell to balance the concentration on both sides of the membrane. Since plasmolysis is the loss of water from a cell, it occurs when a cell is in a hypertonic solution. Conversely, when a cell is placed into a hypotonic solution, there is a lower solute concentration outside the cell than inside, and water rushes into the cell. In an isotonic solution, solute concentrations are the same on both sides, so there is no net gain or loss of water.
Plant cells fare best in hypotonic solutions. This is because when plant cells are full of water, they push against each other to form the basic support structure for the plant and allow it to stand upright. Plant calls full of water are known as turgid cells; they exert turgor pressure on each other. The cells’ rigid cell wall keeps them from bursting. Unlike plant cells, animal cells do not have a cell wall in addition to their cell membrane. When animal cells are placed in a hypotonic solution and too much water rushes in, they will lyse, or burst. They fare best in isotonic solutions instead.
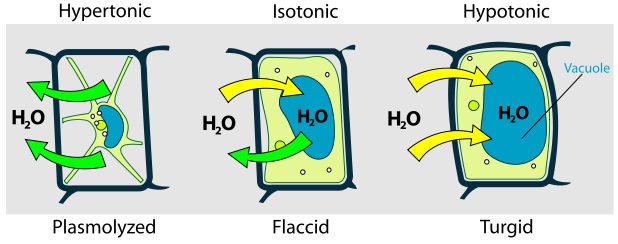
This figure shows a plant cell in different types of solutions:
Types of Plasmolysis
Concave Plasmolysis
Concave plasmolysis is a process that can usually be reversed. During concave plasmolysis, the protoplasm and the plasma membrane shrink away from the cell wall in places due to the loss of water; the protoplasm is then called protoplast once it has started to detach from the cell wall. Half-moon-shaped “pockets” form in the cell as the protoplast peels from the surface of the cell wall. This can be reversed if the cell is placed in a hypotonic solution, which will cause water to rush back into the cell.
Convex Plasmolysis
Convex plasmolysis is more severe than concave plasmolysis. When a cell undergoes complex plasmolysis, the plasma membrane and protoplast lose so much water that they completely detach from the cell wall. The cell wall collapses in a process called ctyorrhysis. Convex plasmolysis cannot be reversed, and results in the destruction of the cell. Essentially, this is what happens when a plant wilts and dies from lack of water.
Defenses Against Plasmolysis
Plasmolysis happens in extreme cases of water loss, and does not happen very often in nature. Plants have a couple mechanisms to protect against water loss. Stomata, which are small holes on the underside of a plant’s leaves, close to help keep water in the plant. Plants also naturally produce wax that is another defense against water loss.
Examples of Plasmolysis
Although plasmolysis more commonly happens in a laboratory setting, it can happen in real-life settings as well. For example, during periods of extreme coastal flooding, ocean water deposits salt onto land. Too much salt causes the water to flow out of any plants on the affected land, killing them. Chemical weedicides are also used to kill unwanted plants through plasmolysis. This same process is also used when a lot of salt and/or sugar is added to preserve food and make jams, jellies, and pickles. The cells lose water and become less conducive to the growth of microorganisms such as bacteria, allowing these food items to be preserved.
Related Biology Terms
- Osmosis – Process by which water diffuses across a membrane to balance out the solute concentration on either side of the membrane.
- Cell wall – Found in plant and fungi cells, a tough layer surrounding the outside of the cell that provides structural support.
- Ctyorrhysis – Permanent and irreversible collapse of the cell wall due to too much water being lost through plasmolysis.
- Protoplasm – The material comprising the inside of the cell; it is called protoplast when it separates from the cell wall through plasmolysis.
Quiz
1. In what type of solution does plasmolysis occur?
A. Hypertonic
B. Isotonic
C. Hypotonic
2. What mechanisms do plants use to defend themselves against plasmolysis?
A. The plants’ stomata close to help keep water inside.
B. The plants produce wax that keep water inside.
C. The plants pump water into their cells through reverse osmosis.
D. Both A and B
3. What type of solution is best for plant cells?
A. Hypertonic
B. Isotonic
C. Hypotonic