Oxidative Phosphorylation Definition
Oxidative Phosphorylation, also known as OXPHOS, refers to the redox reactions involving the flow of electrons along a series of membrane-bound proteins, coupled with the generation of Adenosine triphosphate (ATP).
Oxidative phosphorylation is the fourth and final step in cellular respiration. While respiration can be represented as the simple ‘combustion’ of carbohydrates to form carbon dioxide and water, the cell cannot afford to release all the chemical energy stored in carbohydrates in a single step, since it would irreversibly damage the intricate balance and homeostasis of the organism.
Cm(H2O)n + xO2 -> mCO2 + yH2O + energy
The equation represents the combustion of carbohydrates.
Instead, nutrients are first digested and assimilated. They undergo metabolism in the cytoplasm and their end products are transported into the mitochondria, to participate in the Kreb’s cycle, also known as the citric acid cycle. Here, the chemical energy in the organic molecules is released gradually, through step-wise oxidation to carbon dioxide. The process also generates a number of high-energy electrons, which are harnessed by special molecules called electron carriers. The most common electron carriers associated with oxidative phosphorylation are nicotinamide adenine dinucleotide (NAD+) and flavin adenine dinucleotide (FAD). When NAD+ and FAD accept electrons, they become reducing agents (NADH and FADH2) that are capable of transferring these electrons to molecules that have a high affinity for them.
Oxidative phosphorylation begins with the oxidation of NADH and FADH2. The energy of the electrons released by these two molecules is harnessed in a step-wise manner and used to create a proton gradient across the inner mitochondrial membrane in eukaryotes. This proton gradient then powers the formation of ATP from ADP, catalyzed by the enzyme ATP synthase. The ultimate acceptor of these high-energy electrons is oxygen and therefore oxidative phosphorylation generates both ATP and water.
ATP as Energy Currency
ATP has three phosphate groups in close physical proximity to one another. The addition of every phosphate group needs to be coupled with some other energy-releasing or exergonic reaction, since the natural repulsion between the negatively charged phosphate groups needs to be overcome. Once ATP is formed, it becomes a ready store of energy, because the terminal phosphate bond can be quickly hydrolyzed to power some other process within the cell. This makes ATP behave like the energy currency of the cell.
The presence of ATP allows the cell to conduct respiration, store the energy temporarily in the high-energy bonds of the molecule, and use it at different locations and points in time. Without ATP, every single endergonic (energy-requiring) reaction would have to be coupled in time and space with the oxidation of nutrients, severely limiting the complexity possible within a living organism.
The importance of this molecule is underscored by the fact that ATP is found in all living cells and ATP synthase is largely conserved across the living world. Additionally, any process that fundamentally interrupts the generation of this molecule leads to a very quick death.
Energy Conversion
The original energy source for nearly the entire biosphere is the nuclear reactions within the sun. From plants and other autotrophs, to single celled protozoa and the largest mammals, the energy to sustain life is derived from the sun through a series of energy conversions.
In autotrophs, solar radiation is first used to generate high-energy electrons, which are then used to pump protons against their concentration gradient, creating a proton-motive force across a membrane. The potential energy in such an electrochemical gradient is channeled to generate ATP, which, in turn, facilitates the formation of complex macromolecules. Nutrients created by autotrophs are consumed by heterotrophs, digested and then metabolized within their cells. The chemical bond energy in these molecules behaves like a storage system for the energy initially harnessed from the sun. When nutrients are oxidized, the bond energy is released – both as ATP and as high-energy electrons. In a process that parallels the initial reactions within chloroplasts, these electrons are used to gradually create an electrochemical gradient that, once again, powers the formation of ATP. ATP is repeatedly generated and utilized to sustain the living processes of the organism.
Energy from the sun, therefore, is transmuted from one form to another, as the energy in electrons, the potential energy in proton gradients and the bond energy of macromolecules.
Structure of Mitochondria
The mitochondria is said to have evolved from ancient bacteria that became endosymbionts within eukaryotes, creating the first nucleated cells that could undergo aerobic respiration. The structure of mitochondrial membranes reflects this origin; these organelles have a distinct genome, independent protein translation machinery (tRNA, ribosomes, and associated proteins) and respiratory chain complexes that mirror aerobic respiration in bacteria.
Mitochondria have two membranes – the inner and outer membrane – and the outer membrane is structurally similar to eukaryotic plasma membranes. However, they also contain a number of transmembrane channels called porins. The largest molecules to diffuse freely through porins are about 6000 Daltons in atomic mass, making the membrane permeable to most small molecules and proteins. The inner membrane is impermeable to nearly every molecule with the exception of oxygen, carbon dioxide and water. This property is important, because it allows the organelle to regulate the flow of ions and molecules across the membrane and use the differential concentration of these substances to power the formation of ATP.
The inner mitochondrial membrane has a number of invaginations called cristae that increase its surface area. The protein complexes involved in the redox reactions of oxidative phosphorylation are embedded in cristae. Similarly, ATP synthase is also present as a transmembrane protein on cristae. The inner mitochondrial membrane encloses a protein-rich matrix that receives pyruvate molecules from the cytoplasm and contains enzymes that generate acetyl coenzyme A. The matrix is also the site where the reactions of the citric acid cycle occur. The matrix is so dense that it resembles a protein crystal, with a protein concentration of 500 mg/ml.
The impermeability of the inner mitochondrial membrane to ions allows it to maintain an electrochemical potential of 180 mV generated primarily by the pumping of protons from the matrix into the inter-membrane space. This makes the matrix of the mitochondrion slightly alkaline, with a pH of about 8.
Electron Transport Chain
The electron transport chain of oxidative phosphorylation involves four major protein complexes, (numbered I, II, III and IV) each with increasing reduction potentials. In essence, as the electron moves from one protein complex to the next, it travels towards molecules that have a greater affinity for electrons. The electron loses energy in this process, and this energy is used to pump protons into the inter-membrane space.
Between the two electron carriers, NADH has a lower reduction potential, and releases electrons to complex I. Complex I is also known as NADH:quinone oxidoreductase and is instrumental in the transfer the electrons from NADH to a protein called ubiquinone. This enormous membrane-bound complex consists of 46 polypeptide chains and can combine the acceptance of two electrons from NADH with the movement of 4 H+ ions from the matrix to the inter-membrane space. Each of these four protons is pumped through a separate, dedicated channel.
On the other hand, FADH2 donates its electrons through complex II, which is also known as succinate dehydrogenase. This relatively smaller complex consists of four subunits and does not facilitate the tandem movement of protons across the membrane. Of the four subunits, two act as hydrophobic anchors to the inner mitochondrial membrane. The third subunit has a covalently attached FAD molecule. The four subunits together form a ubiquinone-binding site. Complex II participates in both the citric acid cycle and in oxidative phosphorylation. Therefore, it becomes a parallel route for electrons to reach the quinone pool, and FAD receives high-energy electrons from the products of fatty acid metabolism.
Thereafter, the electrons travel through the same pathway, being fed into complex III through ubiquinone, which acts as a mobile electron carrier within the membrane. Complex III is also involved in pumping H+ ions into the inter-membrane space. From complex III, another mobile carrier called cytochrome C carries electrons to complex IV. Complex IV acts as the site for the final step in these reactions involving the splitting of an oxygen molecule and the formation of water. Water is the final resting spot for the electrons that entered the system through NADH and FADH2 and is either used in the cell’s metabolic pathways or is excreted from the body.

This is a schematic representation of the complexes involved in oxidative phosphorylation and ATP generation.
At the end of the electron transport chain, NAD+ and FAD are regenerated and the electrochemical gradient is created.
ATP Synthase and ATP Generation
The inner mitochondrial membrane is impermeable to ions and has one dedicated channel for the flow of protons back into the matrix. This is the membrane-bound ATP synthase enzyme. It consists of two parts – the F0 and F1 regions. F0 forms the proton pore and is embedded within the membrane. When protons flow back into the matrix from the inter-membrane space, the catalytic activity of the enzyme uses the potential energy released due to chemiosmosis to synthesis ATP from ADP and Pi.
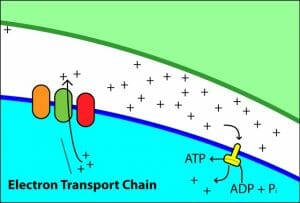
The image is a simplified representation of the proton motive force being used to generate ATP.
Related Biology Terms
- Electrochemical Gradient – A gradient consisting of two parts – a difference in charge and a difference in solute concentrations. Commonly seen across biological membranes.
- Phosphoanhydride Bond – Chemical bonds formed by a dehydration reaction between two phosphoric acid derivatives. Most commonly encountered in the bonds linking the three phosphate groups of ATP or GTP together.
- Redox Reactions – A reaction that involves the oxidation of one chemical species with the concurrent reduction of another chemical.
- Synthases – An enzyme that catalyzes the formation of a single molecule from two components, usually without the direct involvement of ATP.
Quiz
1. Which of these molecules has the highest reducing potential?
A. NADH
B. FADH2
C. Complex I
D. Complex IV
2. Where do the electrons donated by FADH2 enter the electron transport chain?
A. Complex I
B. Ubiquinone
C. Cytochrome C
D. Complex II
3. Which of these is a property of the inner mitochondrial membrane?
A. Impervious to water
B. Impermeable to ions
C. Presence of porins
D. All of the above
