Definition
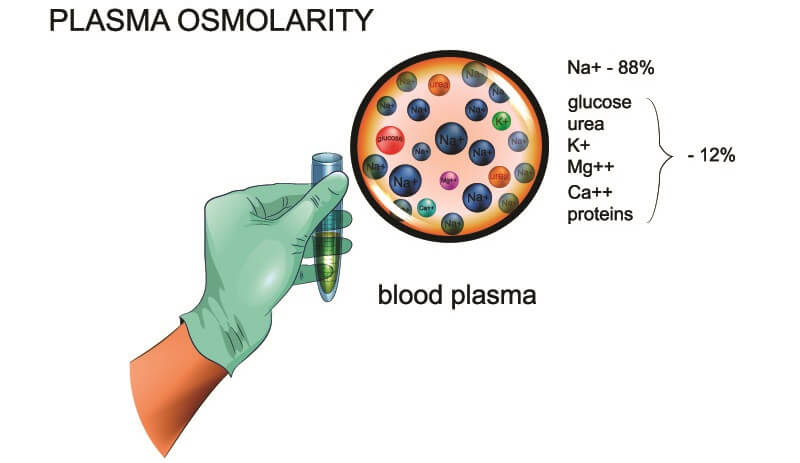
Osmolarity is the total solute concentration within a specific volume of a solvent expressed in osmoles per liter (Osm/L) or milliosmoles per liter (mOsm/L). These solute (dissolved particle) concentrations must be osmotically active – that is, they cause the movement of water across a selectively permeable membrane (the cell membrane) via osmosis. Osmoles are measured concentrations of dissociated ions in a solution that contribute to osmotic pressure.
What is Osmolarity?
To understand osmolarity, we need to know about how water and molecules travel across cell membranes. This requires basic knowledge of several associated terms.
Diffusion
Molecular diffusion is the net movement of molecules in solid, liquid, or gas form from an area of high molecule concentration to low concentration. No energy in the form of adenosine triphosphate (ATP) is required. Solid diffusion is the result of atom movement under the influence of temperature. For this reason, it is rarely seen to be a passive form of molecular transport – solids require energy in the form of heat to give their atoms enough energy to disperse.
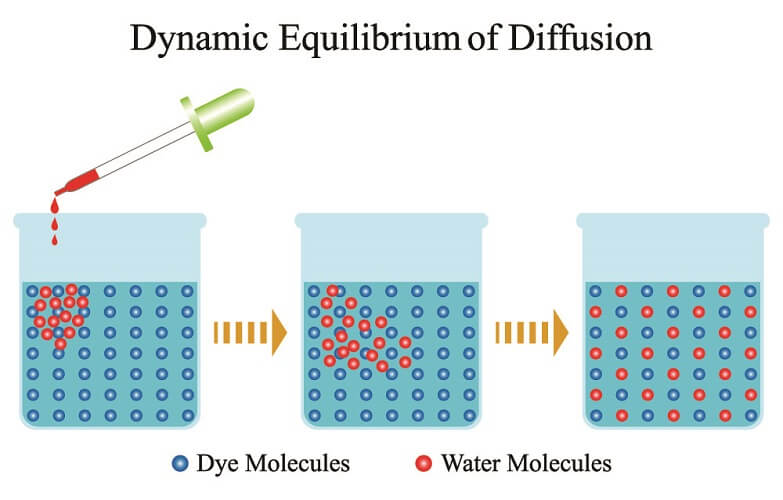
In the human body, diffusion is the passive movement of molecules in water or gas along a concentration gradient – from an area of high concentration to an area of low concentration. The final goal of diffusion is to create the same concentration across connected areas. This can happen in the presence of a sieve-like barrier called a semipermeable membrane or without. In human biology, this membrane is the phospholipid membrane of a cell.
Osmosis is a subcategory of diffusion but, rather than the movement of particles across a concentration gradient, describes the movement of water molecules – always across a semipermeable membrane – from a low concentration of solute toward a higher concentration of the same solute.
Semi-permeable Membrane
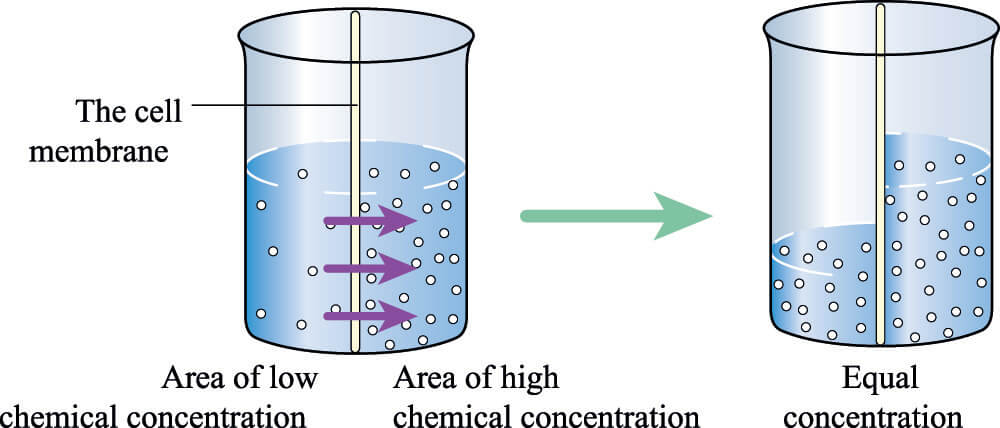
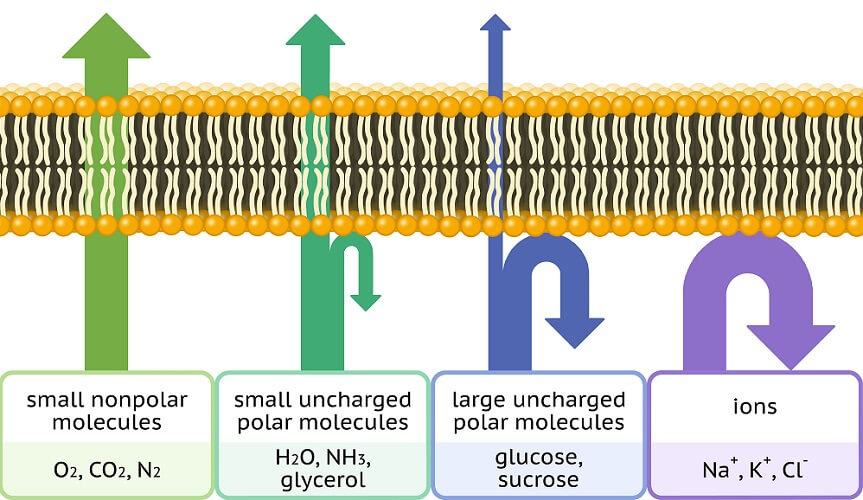
A semi-permeable membrane allows some molecules to pass through but prevents large or charged molecules from doing the same. In osmosis, a semi-permeable membrane is always required. A selectively-permeable membrane is not obligatory for diffusion to occur.
The phospholipid bilayer of a cell membrane has a hydrophobic core (non-polar) and a hydrophilic (polar) inner and outer surface. When larger molecules have an electric charge, such as in the case of ions and polar molecules, they are unable to cross the membrane. Instead, they need open channels (pores) or transport proteins to enter or exit the cell.
Solution
A solution is a mix of solvent and solute.
A solvent – solid, liquid, or gas – can separate other molecules by changing their electrical charges and causing movement. In the body, the solvent is water. Plasma osmolarity concerns the water solvent and countless solutes of the blood.
Water molecules are the driving force of osmosis. In diffusion, water molecules cause solutes to move across an area until concentrations are equal. In osmosis, water moves across a membrane that is not open to the solute and makes solute concentrations similar on either side of that membrane.
The concentrations of solutes and water in the blood plasma and extracellular fluid are very similar; however, concentrations inside and outside the cell membrane can be very different. This is where osmosis has its primary function.
Osmosis
Osmosis is the movement of a solvent – not a solute – through a semi-permeable membrane across a concentration gradient. In the body, it refers to the concentration of water molecules on either side of the selectively-permeable cell membrane.
The reason water molecules are responsible for osmosis and not the solutes dissolved in it is because many solutes are unable to cross the phospholipid bilayer of the cell membrane. Water molecules can.
For example, sugar molecules are too large to pass through a cell membrane and require active transport. If ten sugar molecules are dissolved in twenty water molecules outside a cell and two sugar molecules are dissolved in one hundred water molecules inside a cell, the concentrations of these two solutions are not the same. By bringing more water molecules into the cell, sugar concentrations become equal and energy is not wasted transporting the bigger glucose molecules.

Isotonic Solution
An isotonic solution is the goal of both osmosis and diffusion. This term describes the same concentration of solutes across two areas and is called a homogenous mixture. Cell membranes continuously allow water and solute molecules to pass from the inside of the cell to the outside and vice versa. This occurs in response to changing solute concentration levels. The transport methods that do not use energy (passive transport) are the most efficient. Osmosis and diffusion are forms of passive transport.
Hypotonic Solution
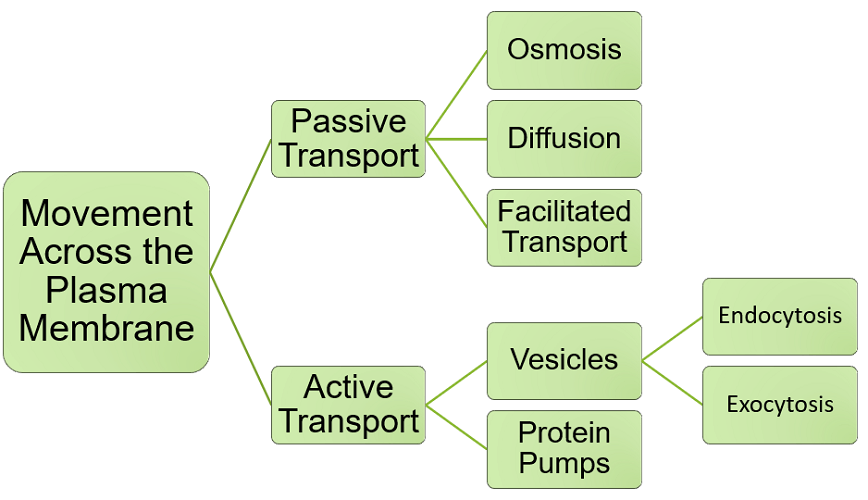
A hypotonic solution describes a lower concentration of a solution outside the cell than inside. If twenty glucose molecules are dissolved in twenty water molecules in the cell cytosol (a ratio of 20:20 or 1:1) and twenty glucose molecules are dissolved in one hundred water molecules in the extracellular fluid (a ratio of 20:100 or 1:5), the solution concentration is lower outside the cell.
Let us imagine that forty water molecules move from the extracellular space into the cell via osmosis. The extracellular ratio of glucose to water becomes 20:60 or 1:3. Adding water to the inside of the cell affects the concentration level there, too. Twenty glucose molecules dissolved in sixty water molecules inside the cell (20:60 or 1:3) creates an isotonic solution on either side of the cell membrane. However, this is not always ideal. Too much water inside the cell can cause it to become turgid; it could burst and die.
Hypertonic Solution
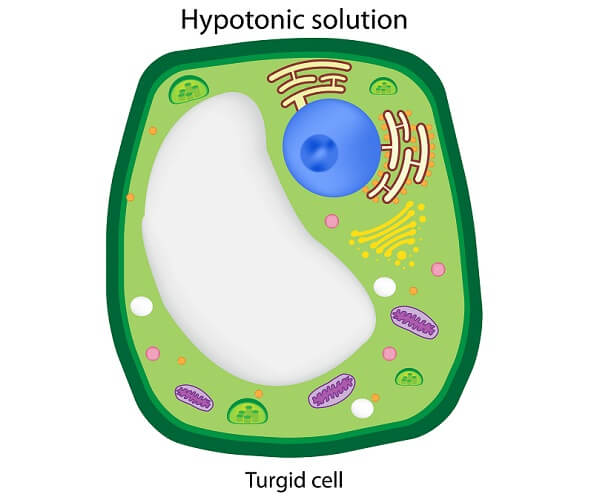
In an extracellular hypertonic solution, water will exit the cell to lower high concentrations outside the membrane. The aim is to produce an isotonic solution on both sides. In a hypertonic solution, the concentration of solutes in a solution is higher outside the cell.
The opposite effect to a hypotonic solution occurs within the cell. It becomes dehydrated through water loss and can lose its ability to function and perhaps die.
Molarity
Molarity is a measurement that expresses the number of moles of a solute per liter of a solution.
A mole is a unit of measurement calculated according to the International System of Units. It is based upon the number of carbon atoms in twelve grams of carbon-12.
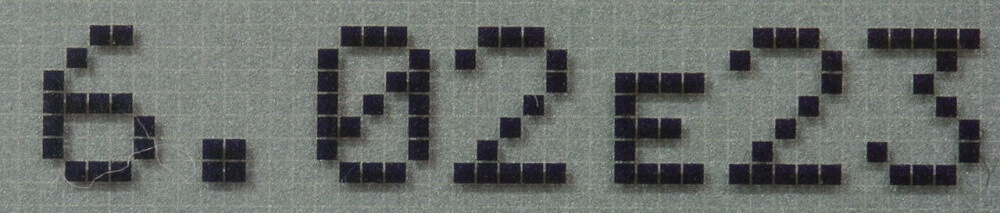
The International Union of Pure and Applied Chemistry has since changed the old formula; carbon atoms have been replaced by the mathematical Avogadro constant of 6.02214076 x 1023 (602,214,076,000,000,000,000,000). For scientists, this change makes little difference; the Avogadro constant just happens to equal the number of carbon atoms in twelve grams of carbon-12.
If we simply looked at the mass of an atom, every number would be tiny and very difficult to calculate. By defining one mole of any substance as having the same mass as twelve grams of carbon-12, calculations become much simpler. The mole helps us to convert elements and combinations of elements into a single unit of mass.
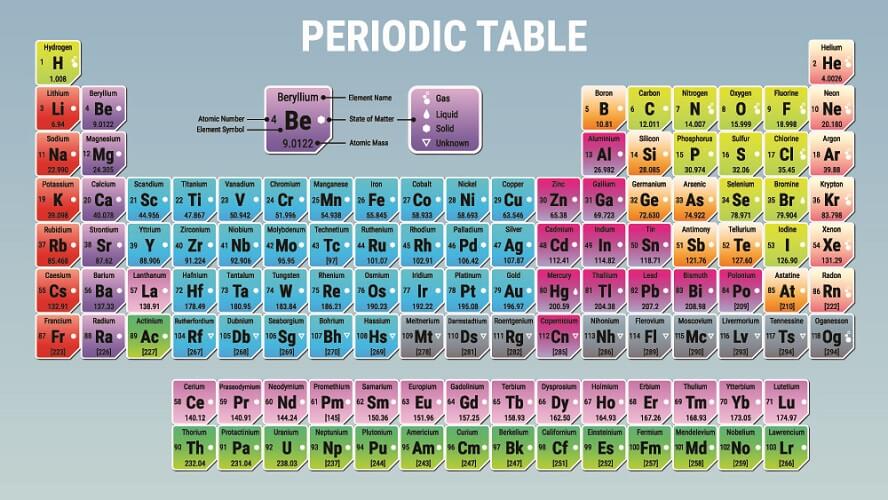
You can calculate moles by looking at the atomic weight of an element. Atomic mass is represented on most periodic tables. To calculate the amount of a substance in moles we look at the elements within that substance individually.
Osmoles
Osmoles are units of osmolarity. Calculations are not based upon the Avogadro constant but according to the different particles of a solute dissolved in water. It is expressed as Osm/kg of water.
Many solutes dissociate in water and only dissociated solutes have osmole units that differ from mole units. When we add salt (NaCl) to water, the positive sodium ions and negative chloride ions split apart. This gives us two different particle types. When you have a one-mole solution of salt in water, you have an osmotic concentration composed of two particles – one sodium and one chloride ion; every mole of sodium chloride is two osmoles in solution.
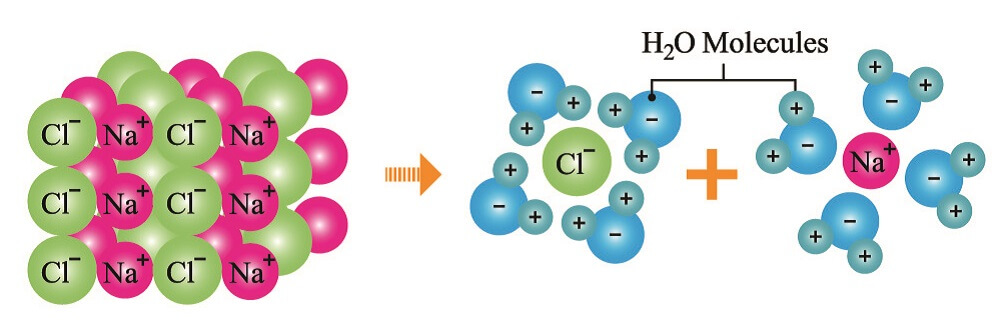
Osmolarity Calculation
Knowing how to calculate osmolarity should be a much simpler task when you are familiar with the above information. We know that an osmolarity definition describes the number of solute particles in one liter of solvent (water).
Osmolarity Calculation Example
Imagine you have 400 ml of water in which 60g of magnesium chloride (MgCl2) is dissolved.
Looking at the periodic table, you note down the atomic mass for magnesium (24.3) and chloride (35.5). You can then calculate the weight in grams of one mole of magnesium chloride: 24.3 plus 35.5 plus 35.5 = 95.3 g.
To convert from grams into moles, divide the number of grams in the solution by the total atomic mass of the solute. Dividing 60 g by 95.3 g gives 0.63 moles of magnesium chloride in a 400 ml solution.
To find the number of osmoles per mole in this solution, look at the total number of particles in dissociated magnesium chloride. As a salt, this compound dissociates into ions. One magnesium and two chloride particles per molecule means that each mole of MgCl2 is three osmoles.
To calculate how many osmoles can be found in your 400 ml solution, multiply moles (0.63) by osmoles (3). There are 1.89 osmoles of solute in 400 ml of water.
However, an osmolarity formula result is expressed in osmoles per liter. Now you need to divide 1.89 osmoles by 0.4 liters (400 ml) to arrive at the answer – the osmolarity of this solution is 4.73 Osm/L.
When you know the osmolarity of two solutions on either side of a semipermeable membrane, you can calculate the direction of osmosis. Water moves toward the side that has a higher osmolarity. It moves from the most dilute side to the most concentrated side. The side with the high osmolarity is hyperosmotic to the other (hypo-osmotic) side. When concentrations are the same, they are iso-osmotic.

Osmolarity vs Osmolality
Osmolality is another measurement of solute osmoles but in a kilogram of solvent rather than a liter of solvent (osmolarity). While solutes are measured by weight or mass, solvents are measured according to weight (kilograms) or volume (liters). Osmolarity is expressed in Osm/L and osmolality in Osm/kg. As water can change in volume according to temperature, many scientists prefer osmolality. A kilogram remains a kilogram no matter what the temperature; however, a liter of water and a liter of ice do not take up the same amount of space.
Osmolarity of blood plasma is not a test – plasma osmolality is. This measures the concentration of dissolved particles in the blood according to blood weight. Results are given in milliosmoles per kilogram (mOsm/kg).

Osmolarity vs Tonicity
How permeable a cell membrane is and what the solute concentrations are on either side have an influence on the tonicity of an extracellular solution. How much pressure a cell membrane can withstand and how well it pumps water out also influences how much extracellular fluid enters or leaves the cell. We have already looked at hypotonic, isotonic, and hypertonic solutions – these are levels of solution tonicity. Osmolarity primarily depends on the tonicity of the fluid environment but tonicity does not dependentirely on osmosis.
Osmolarity vs Molarity
Osmolarity is the number of solute osmoles in one liter of solution. Molarity is the number of solute moles in one liter of a solution. As many substances dissociate in water, it is often more insightful to calculate using osmoles. However, where a solution does not contain dissociated molecules, such as glucose in water, each mole of solute is also one osmol.
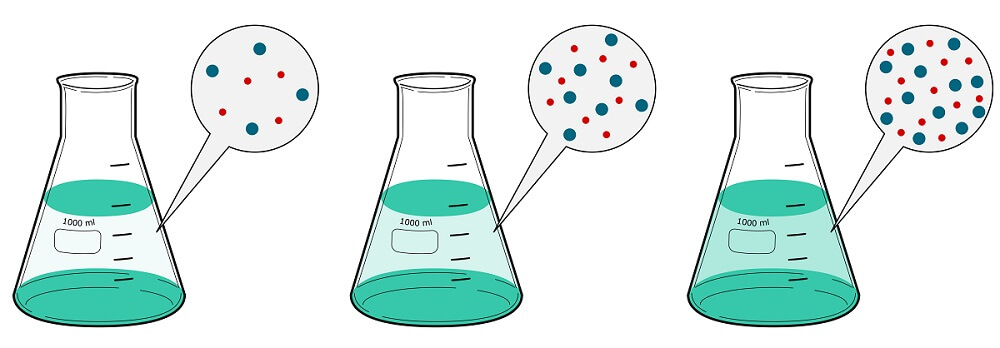
The difference between osmolarity and molarity is explained by the van’t Hoff factor – the number of moles (not the mass or weight) of dissociated solute particles (ions) in a solute.
For example, a 1 mol/L glucose solution does not dissociate; the van’t Hoff factor is, therefore, one. A solution of 1 mol/L glucose (molarity) has an osmolarity of 1 Osm/L.
However, 1 mol/L solution of calcium chloride (CaCl2) dissociates into three ions. It contains one mole of calcium ions and two moles of chloride ions. The 1 mol/L solution (molarity) multiplied by a van’t Hoff factor of three separate particles means 1 mole CaCl2 is 3 Osm/L.