[LS1-7] Cellular Respiration and Energy
This standard focuses on the process of cellular respiration, and how it utilizes stored energy in sugar molecules to fuel cellular processes.
Resources for this Standard:
Here’s the Actual Standard:
Use a model to illustrate that cellular respiration is a chemical process whereby the bonds of food molecules and oxygen molecules are broken and the bonds in new compounds are formed, resulting in a net transfer of energy.
Standard Breakdown
Let’s break down this complex standard into bite-sized pieces:
Energy is Stored in Bonds
The basic level of scaffolding when teaching this standard is based on the concept that energy is stored in bonds. In order to keep the electrons circling around both atomic nuclei, there must be a certain amount of energy within the molecule to keep the electrons flying faster than usual. Otherwise, the molecules would degrade into individual atoms quickly.
Food Contains Energy Stored in Bonds
Sugar is the main energy source for most cells, though there are pathways to process lipids and proteins into energy as well. However, sugar (specifically glucose) is the main energy-storage molecule produced by plants during photosynthesis.
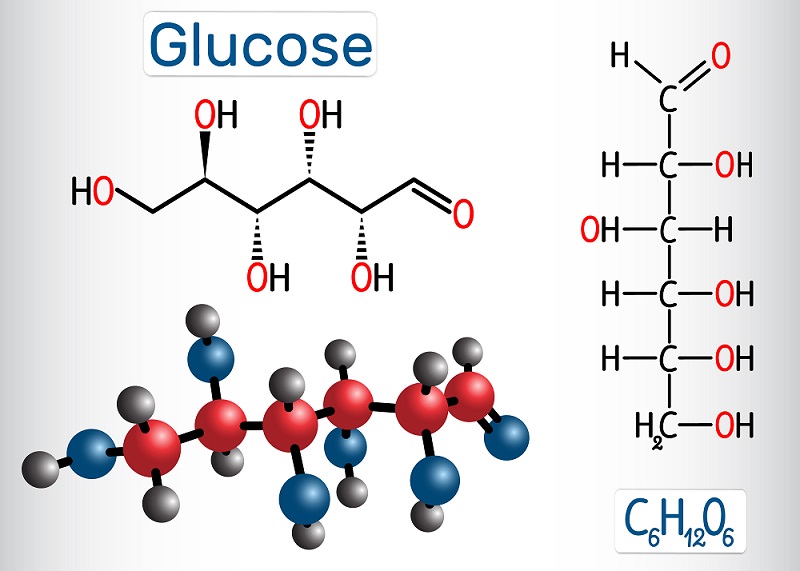
Glucose has many stable bonds, and cells can use glucose to store energy for a long time. To get at this energy, cells use the process of cellular respiration to create ATP. ATP (adenosine triphosphate) can store energy within phosphate bonds, which can activate and energize many cellular proteins and reactions. There are many types of cellular respiration. Most organisms use aerobic respiration (uses oxygen), although there are a number of organisms that use fermentation (a type of oxygen-free ATP creation) and anaerobic respiration (similar to aerobic respiration but with a different molecule than oxygen to accept electrons during the process).
The main process that students should be familiar with is aerobic respiration.
Steps of Aerobic Respiration
Aerobic respiration – and all other forms of respiration – start with the process of glycolysis. This process splits a glucose molecule into two smaller pyruvate molecules. Glucose has 6 carbon molecules, and through the process of glycolysis, two 3-carbon pyruvate molecules are produced. This process also creates a small amount of ATP, which will help power the remainder of the process. It also creates NADH, a molecule that essentially carries electrons to the electron transport chain – aptly called an “electron carrier”.
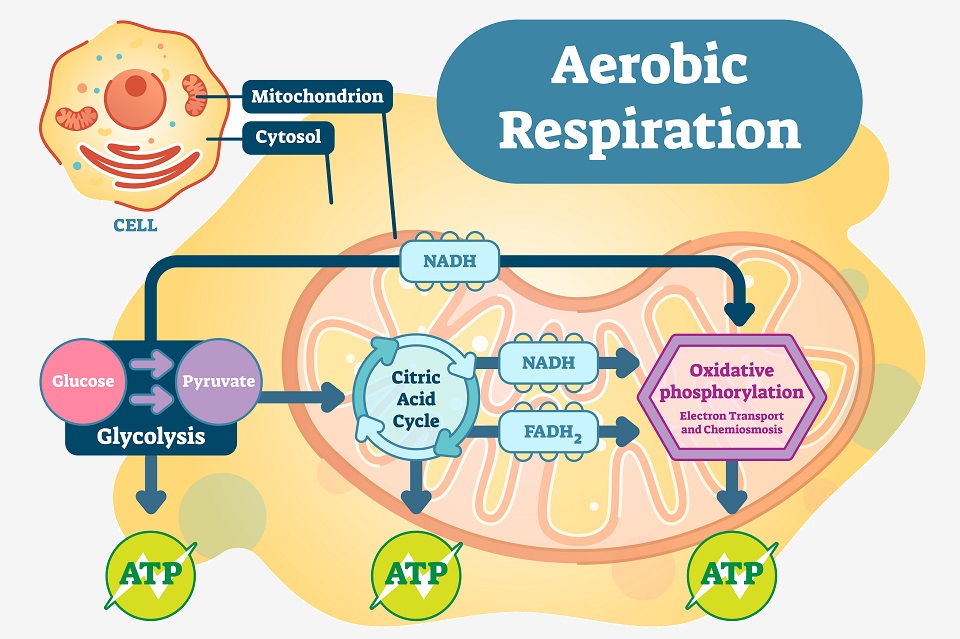
After glycolysis, the pyruvate molecules make their way into the mitochondria. Within the inner mitochondrial matrix, the Citric Acid Cycle takes these pyruvate molecules and slowly extracts the electrons from the bonds. Small amounts of ATP are created, in addition to many “full” electron carriers. These electron carriers move to the folds of the mitochondria, depositing their electrons into the Electron Transport Chain.
This final chain of proteins is where the most ATP is created. A series of specialized proteins within the membrane of the mitochondria accept and transfer electrons. Using the energy they give off as they are transferred, H+ ions are moved from the inside to the outside of the inner membrane. This builds up ions in the intermembrane space, creating a large gradient of powerful electrochemical energy. However, this chain must have an “end” where it can dump the electrons.
Oxygen is Needed to Collect Electrons
At the end of the Electron Transport Chain, electrons need to exit the system so new electrons can enter and continue the proton pump action. Therefore, something needs to serve as the final electron acceptor. Luckily, O2 makes a perfect electron acceptor. Upon receiving two extra electrons, the oxygen molecules break apart. They quickly bind with two free hydrogen ions to form H2O – water!

The electrons can be seen moving through the Electron Transport Chain in the above image (the purple arrows). Ultimately, the Electron Transport Chain uses the energy gained by breaking apart glucose molecules in previous steps of respiration in order to push H+ ions to one side of the mitochondrial inner membrane. The ions build a chemical gradient, which is used to activate ATP synthase – the large protein complex on the right in the image creating ATP by releasing the energy from the hydrogen ion gradient. Because oxygen is used in this complex scheme to add phosphate groups to ADP molecules, this process is called “oxidative phosphorylation.”
A little clarification:
The standard contains this clarification statement:
Emphasis is on the conceptual understanding of the inputs and outputs of the process of cellular respiration.
So, you should focus on these big ideas for this standard:
Aerobic Respiration
Aerobic respiration is essentially the reverse process of photosynthesis. Where photosynthesis combines water, carbon dioxide, and energy – aerobic respiration releases energy, water, and carbon dioxide. The molecule that sits between these two processes is glucose.
Glucose (sugar) acts as an energy storage molecule. Plants export sugar from the leaves and send it all the way down to the roots. This allows all regions of the plant to use energy harvested by the chloroplasts in the leaves. Herbivores eat these sugars to power cellular growth. Carnivores, though they don’t eat the plant sugars directly, are eating the sugar energy after it has been used to create new cells and tissues in herbivores.
Fermentation and Anaerobic Respiration
However, aerobic respiration is just one of many ways that organisms can release the energy stored in sugar. Many environments – such as the bottom of a lake or inside of a sealed container – do not get well oxygenated. In these environments, organisms must have different methods of releasing the energy bound in organic molecules.
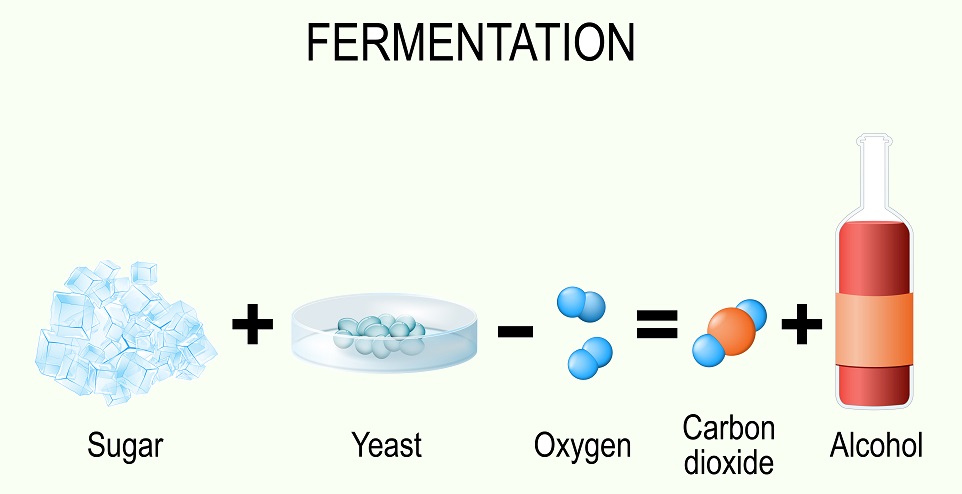
One common method used by many organisms is fermentation. During fermentation, the end products of glycolysis are converted into other substances. Fermentation essentially uses pyruvate (the end product of glycolysis) to accept electrons stored on electron carriers. Since the Electron Transport Chain cannot function, electron carriers get full of electrons during glycolysis and these electrons are transferred to pyruvate to recycle the carriers. Several different methods of fermentation are known – from alcohol fermentation by yeast to the lactic acid fermentation human muscles undergo when they are low on oxygen.
By contrast, anaerobic respiration still utilizes the Electron Transport Chain. Instead of oxygen (because there is none), other molecules are used as the final electron acceptor. Anaerobic respiration, therefore, is just often more productive than fermentation because electron carriers can contribute their energy to the very efficient proton pump ATP-creation mechanism.
What to Avoid
This NGSS standard also contains the following Assessment Boundary:
Assessment should not include identification of the steps or specific processes involved in cellular respiration.
Here’s a little more specificity on what that means:
Steps and Specific Processes:
This boundary intends to keep learners in the conceptual space. It is far more important, from a scaffolding standpoint, that the concept and general mechanisms of cellular respiration are learned over the small number of specific biochemical processes that could be memorized in the same amount of time. There’s plenty of time in college to memorize the exact biochemical mechanisms of the process.
