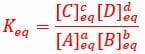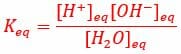Equilibrium Constant Definition
An equilibrium constant, Keq, is a variable that describes a chemical reaction’s tendency to proceed to completion, meaning all the reactants are converted to products. The equilibrium of a reaction is the point at which the conversion of reactants into products equals the conversion of products back into reactants.
Equilibrium Constant Overview
A large equilibrium constant means that the reaction proceeds in the forward direction, from reactants to products, until almost all the reactants have been converted to products. A small equilibrium constant, or when Keq is less than one, means that the chemical reaction will favor the reactants and the reaction will proceed in the opposite direction. An equilibrium constant of 1 indicates that the reactants and products will be equal when the reaction reaches equilibrium.
Scientists use the equilibrium constant of an equation to better understand how quickly the equilibrium will be reached, and whether the equilibrium will favor reactants or products. The constant can be calculated using the ratio of products to reactants when the equation has reached equilibrium. The equilibrium constant is often represented by the variable Keq, which is defined by the equilibrium constant expression seen below.
Equilibrium Constant Expression For a Reaction
The equilibrium constant expression describes the concentration of products divided by the concentration of reactants when the reaction reaches equilibrium. This expression can be seen below.
In the reaction: aA + bB <=> cC + dD
Each term describes the concentration of a reactant or product in the reaction where chemicals A and B combine to produce products C and D. The lowercase letters indicate the number of moles of each chemical. The brackets around a letter, [A], indicate the concentration of each chemical, and the subscript denotes that the equilibrium constant is determined by the concentration of each molecule at equilibrium.
J. Willard Gibbs, a famous scientist who studied the energy present in reactions, showed that the equilibrium constant was directly related to the amount of free energy change that occurs during a reaction, denoted ∆G. Gibbs showed that every reaction has a standard free-energy change, or ∆G°. While the total free energy change of each reaction is also governed by the initial concentrations of chemicals, the standard free energy is calculated with the equation below, using the equilibrium constant of the equation.
∆G° = -RTln(Keq)
This equation shows that the standard free energy change is simply another way of describing the driving forces of a reaction, and which way they will proceed. While the equilibrium constant tells us whether we will have more reactant or products at the end of a reaction, it does not hint at how fast this reaction would take place. This is known as the rate constant and is denoted by a lowercase k. The rate constant is related to a variety of other equations related to the speed at which reactions happen. The equilibrium constant is important to a number of biological reaction, as seen in the examples below.
Examples of Equilibrium Constant
Ionization of Water
Water is the basis for all life on Earth. One of the main reasons water is such a good solvent is its ability to form hydrogen bonds with both itself and non-water molecules. Not only does this ability allow water to dissolve and diffuse solutes, but also allows water to carry an electrical current. When water, H2O, forms hydrogen bonds, the hydrogen is pulled away from the oxygen and the molecule disassociates into a hydrogen ion (H+) and a hydroxide ion (OH–).
Individual hydrogen protons rarely exist freely in solution, and immediately forms a bond with water molecule it was hydrogen bonded to. This forms a hydronium ion, or H3O+. The equilibrium constant for this reaction is, therefore, the concentration of hydrogen ions and hydroxide ions, divided by the concentration of normal water molecules, as seen below.
The equilibrium constant of this reaction can be measured by the electrical conductivity of water, which is determined by the concentration of (H3O+). Hydronium ions pass an electrical signal in the form of the transfer of electrons, which can be measured by sensitive electrical equipment. Thus, the equilibrium constant of water has been measured by sensitive electrical equipment to be 1.8 x 10-16, meaning the water has a much higher probability of being the reactant H2O, as opposed to becoming the hydronium ion. The process can be seen in the image below.
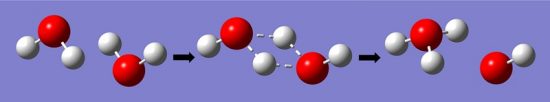
Cells, Free Energy, and the Equilibrium Constant
Although the equilibrium constant is measured when a reaction is at equilibrium, this does not mean that all reactions are allowed to proceed to equilibrium. In the cell, many reactions are constantly resupplied with various chemicals, which keeps the reactions of the cells far from equilibrium. The equilibrium constant, however, describes the tendency of these reactants to form products. Some reactions are exergonic, meaning they release energy when they happen. These reactions have a high equilibrium constant, describing their tendency to become products.
These reactions can also be said to have a positive change in free energy, meaning they give off energy to reactions around them. Other important reactions are endergonic, meaning they require energy to take place. These reactions have a low equilibrium constant, describing their tendency to remain as reactants. Cells couple these reactions to allow the endergonic reactions to take place. This can be seen in many typical cellular reactions that use the high equilibrium constant of ATP converting to ADP to drive endergonic reactions, such as the formation of proteins or fatty acids.
Quiz