Dynamic equilibrium is the steady state of a reversible reaction where the rate of the forward reaction is the same as the reaction rate in the backward direction. Static equilibrium, also known as mechanical equilibrium, means the reaction has stopped. In other words, the system is at rest. In biology, the equilibrium of a system is called homeostasis.
Changes in temperature, pressure, the addition of more reactants/products and changes in other variables cause a system to create a new point of equilibrium. This effect is explained by Le Chatelier’s principle.
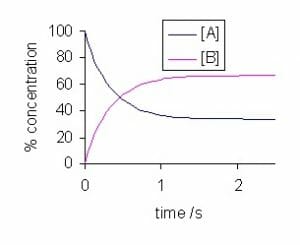
The graph above shows that the reactant (A) and the product (B) are in dynamic equilibrium when the lines are parallel over time. If the Y-axis is changed to rate, the two parallel lines would overlap, indicating that forward and backward reactions are proceeding at the same rate.
Reference
- Difference Between Static and Dynamic Equilibrium. (November 15, 2015). Retrieved from http://pediaa.com/difference-between-static-and-dynamic-equilibrium/
