Definition
Creatine phosphokinase (CPK), or simply creatine kinase (CK), is an enzyme that helps regulate the concentration of adenosine triphosphate (ATP) within a cell. To do so, creatine kinase catalyzes the movement of a phosphate group from ATP to creatine, forming phosphocreatine. This molecules stores the phosphate group in a stable form, acting as an energy reservoir in cells. Cells that have high ATP requirements have higher levels of creatine kinase.
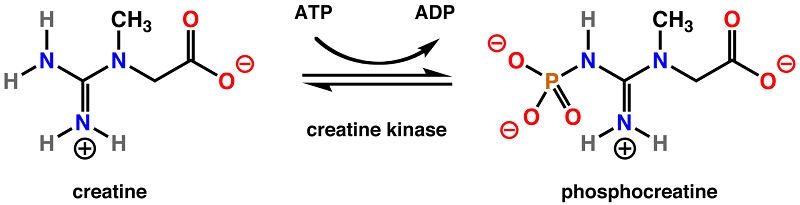
Overview of Creatine Kinase
In cells which need a lot of ATP, it is more economical to store the ATP as a less reactive molecule until it is needed. Thus, the phosphate group is transferred from ATP by creatine kinase to a creatine molecule. The end products are adenosine triphosphate (ADP), and phosphocreatine. This reaction is reversible, and when ATP is needed, it can easily be regenerated by the enzyme from the stored pool of phosphocreatine.
Phosphocreatine is much more stable than ATP, and cannot be directly used by most enzymes. Instead, creatine kinase must reverse the reaction to move a phosphate group back to a molecule of ADP to form more ATP, the energy currency of the cell.
As a result, muscle cells, cardiac cells, and other cells with a high demand for energy can store the energy without disrupting the equilibrium of ATP. This allows the cell to continue producing ATP and storing the extra energy for when it is needed.
Creatine Kinase Levels in the Blood
The level of creatine kinase within the blood is measured in units of enzyme activity (U) per Liter of blood (L), or U/L. A typical range for healthy individuals is between 20 and 200 U/L.
Clinically, the creatine kinase level in the blood is a measure of damage of tissue enriched with the enzymes. For example. the enzyme is elevated in the case of muscle or heart damage. This could be as a result of a heart attack, muscle injury, intense exercise, muscle necrosis, kidney injury, or neuromuscular disorders. Thus, creatine kinase levels are often used as a biomarker for cardiac events or muscle damage.
The levels of creatine kinase in these cases can be as high as 50,000 – 200,000 U/L, or even higher during exceptional cases of severe muscle breakdown (rhabdomyolysis).
Creatine Kinase Function
Creatine kinase is found primarily in tissues which require a lot of ATP. Muscle cells, nerve cells, and even sperm cells are examples of highly active cells which contain large amounts of the enzyme. This is because these cells must use a large amount of ATP to carry out their functions.
Because of the nature of ATP, it must remain within tightly regulated concentrations to preserve the function of various biochemical pathways. Therefore, the energy of ATP must be held in another place until it is needed. This reservoir is phosphocreatine, which is simply a creatine molecule attached to a phosphate group.
The energy held in this phosphate group can be efficiently and quickly converted by creatine kinase either to or from ATP. When there is too much ATP, the enzyme functions to lower the concentration by converting ATP to ADP. It stores the extra phosphate in a creatine molecule, creating phosphocreatine. The pool of phosphocreatine in the cell is much larger than the amount of readily available ATP. For this reason, it is considered a “fuel tank” or energy storage system.
As the mitochondria produce ATP through respiration (specifically through oxidative phosphorylation), the energy transfers to phosphocreatine molecules, which relocate to the cytoplasm of the cell. The cytoplasmic creatine kinase can then release the energy stored in phosphocreatine by regenerating ATP, allowing the cell to harness this chemical energy.
Therefore, creatine kinase maintains the energy reservoir, efficiently saving up ATP. This forms what is known as the phosphocreatine circuit, and is essential for regulating cellular homeostasis.
Structure of Creatine Kinase
Creatine kinase, like all proteins, is a polypeptide chain, made up of a specific sequence of amino acids. When folded properly, this chain takes on a three-dimensional form, which gives it the ability to interact with certain molecules.
The structure of creatine kinase provides it with an affinity to both creatine and phosphocreatine, allowing it to catalyze their respective conversions. Another site on the enzyme is dedicated for interaction with ATP and ADP. As both molecules attach to the enzyme, it will either take a phosphate group from ATP and add it to creatine or take a phosphate group from phosphocreatine and transfer it to ADP. The end result is either the creation or usage of ATP.
There are multiple types of creatine kinase, coded by different genes. While these forms of creatine kinase differ in their amino acid structure, their function remains similar. However, slight changes in their structure and function allow the creatine kinase to operate in different environments.
Isoenzymes
Creatine kinase is a dimer (it is made up of two subunits that join together to create one functional enzyme). There are two different forms of these subunits, the B type and the M type. The B type stands for ‘brain type’ because this subunit is most often associated with the brain. The M type stands for ‘muscle type’ because this subunit is most often associated with skeletal muscle.
Therefore, there are three different creatine kinase isoenzymes that form as a result of different combinations of these subunits: an enzyme with two muscle subunits (CK-MM), an enzyme with two brain subunits (CK-BB), and an enzyme with one of each (CK-MB). These enzymes are found in the cytoplasm of cells and show tissue-specific localization. CK-BB is found mostly in the brain, CK-MM is found mostly in the skeletal muscle, and CK-MB is found in the cardiac muscle.
There are also two forms of the enzyme found in only in the mitochondria (CK-Mt). Ubiquitous CK-Mt is nonsarcomeric, and is found in various tissue like the brain, smooth muscle, and sperm. Sarcomeric Mt-CK is expressed only in the cardiac and skeletal muscle.
The different forms of creatine kinase all perform the same function but under different conditions. These different forms are necessary to manage the energy reservoir in many different types of cell. In most cells, this reservoir of phosphocreatine is maintained at a concentration much higher than that of ATP. This makes it possible to carry out many energy-draining tasks.
Creatine Kinase Test
The different forms of creatine kinase make it a useful diagnostic tool. Like other enzymes, creatine kinase is leaked into the bloodstream when a cell becomes damaged. If many cells are damaged at the same time, a detectable level of the enzyme (and others) are found in the blood. Doctors can determine which form of the enzyme is in the blood, which can give them clues as to which organs are being damaged.

A serum creatine kinase test can detect many conditions, such as a heart attack, muscle breakdown, and even autoimmune diseases which are attacking certain organs and tissues. For instance, after a heart attack, the level of enzyme rapidly spikes in the blood. Therefore, a creatine kinase test can be used to determine whether symptoms of chest pain are linked to heart muscle damage.
The test can determine that it is a combination of muscular and brain creatine kinase that is present in the blood (CK-MB) This is evidence of damage to the heart. Additionally, because the enzyme rapidly disappears from the blood, it can be used as an indicator to determine when a damaging event happened in the system. This can help find the cause of major cardiac problems.
Quiz
