Definition
A covalent bond or molecular bond is a chemical link between two atoms where electron pairs are shared. Covalent bonds form between two non-metal atoms and non-metal compounds that possess the same or similar values of attraction (electronegativity). Electron pairs shared in a covalent bond are known as shared pairs or bonding pairs and increase the stability of the individual atoms as well as any molecule or compound they may form.
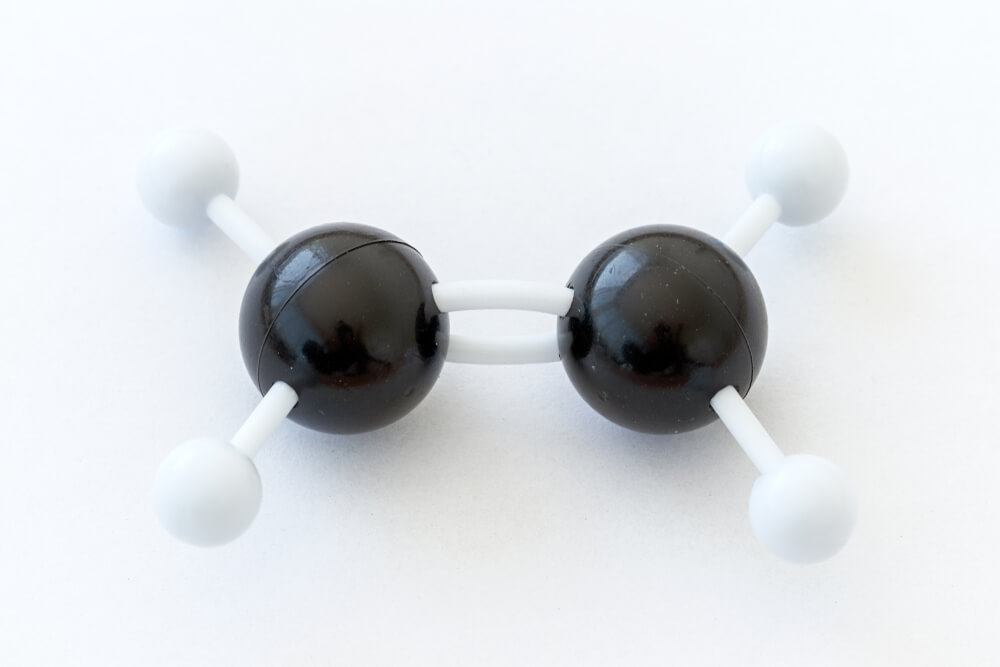
Covalent Bonds between Atoms
Atoms and molecules share covalent bonds so that they become more stable and are less likely to react. Any atomic reaction requires energy and the trick is to use as little energy as possible whenever possible. All atoms are composed of electrons, protons, and neutrons. Proton and neutron particles are found inside the nucleus of each atom and the protons give this part of the atom a positive charge. Neutrons are neutral and don’t affect the positive proton charge. One or more electron shells surround the nucleus – these contain negatively-charged electrons. Electron shells can be described as a cloud-like area surrounding the atom nucleus.
Most atoms of the periodic table are quite stable. They have balanced positive and negative charges that are provided by equal numbers of protons and electrons. However, these atoms can also be found in different, not so balanced forms. Isotopes, for example, are close-copies of elements but have too many neutrons. The presence of excess neutrons means these elements are radioactive. Excess particles must be released (radioactivity) until a more stable environment is achieved. This shows how important it is for any atom to feel balanced – with positive and negative charges being as similar as possible.
Ions are atoms with either too few or too many electrons. This upsets the balance between protons and electrons and creates positively or negatively charged atoms known as ions. Examples of ions are Cl– and H+. In these examples, the chlorine ion (Cl–) has an excess of electrons, creating a negatively-charged ion or anion. The hydrogen ion (H+) has too few electrons and the higher number of positively-charged protons produce a positively-charged ion or cation. A hydrogen anion (H-) is also possible when hydrogen captures another electron. Ions do not form covalent bonds but ionic bonds. When you see images of atomic bonds where an electrical charge is shown, you will know that this is not an example of covalent bonding.
The Basics
Covalent bonds depend on the number of electrons in the outer shell(s) of an atom. How many outer shells an element has depends on the number of electrons that atom has. The simplest element, hydrogen, has a single outer shell because it only has one electron. Sodium metal has three electron shells; the first shell, closest to the nucleus, contains two electrons, and a second, outer shell hosts one electron.
Don’t worry, this isn’t something you have to memorize for every element. Luckily, all atoms have to follow the same rules. This means that the first shell of every atom can provide space for a minimum of one and a maximum of two electrons. There are only two atoms with a single shell – hydrogen and helium. These are great examples as they quickly show how the periodic table can help us to figure out how an atom will react. Hydrogen is found at the left of the periodic table which tells us it is quite reactive – it is always looking for another electron to fill up its shell. Helium, to the right of the periodic table, has a full set of electrons in its single shell. It is part of the inert gas groups and is very unlikely to react (without help) with another atom. This first shell – the closest shell to the atom nucleus – is most commonly referred to as the K-shell.
The second shell – the L-shell – offers place for up to eight electrons. The third shell, not surprisingly called the M-shell, provides space for a maximum of eighteen electrons. The fourth, fifth, and sixth shells host up to 32, 50, and 72 electrons respectively. The higher the number of shells, the greater the energy – this is because the outer electrons must travel and orbit much further away from the nucleus. These outer electrons are responsible for bonding as the positively-charged nucleus has much less of an influence the further away an electron is from it. The diagram below shows us how electrons orbit the atom on four electron shells.
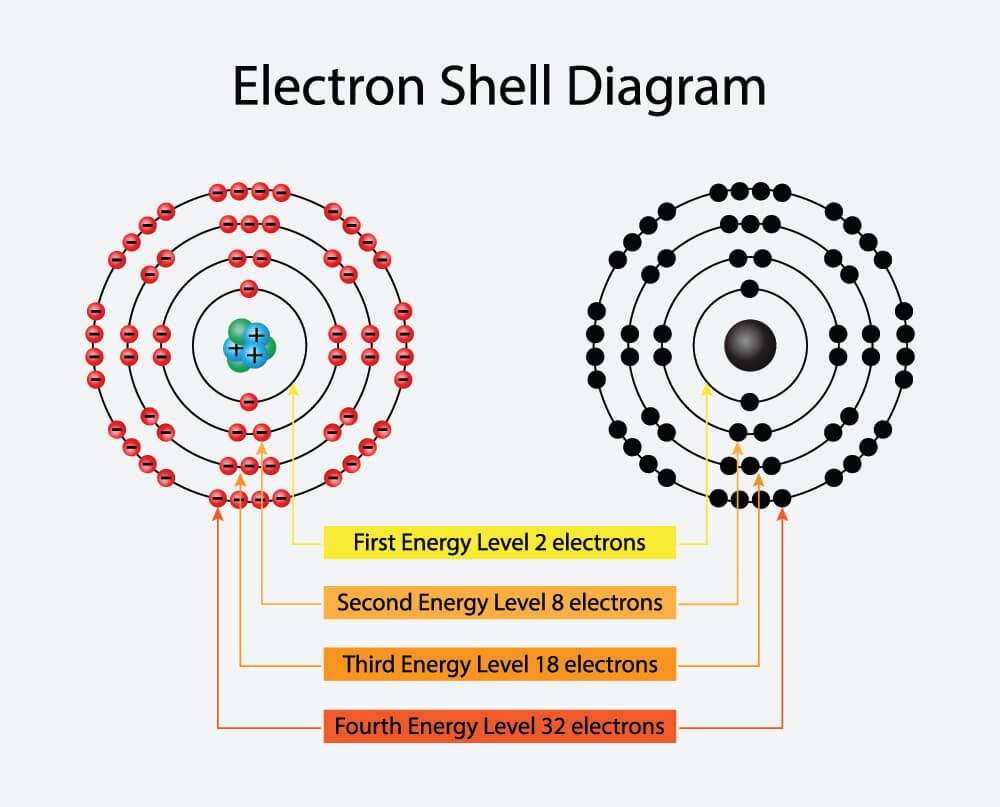
Valence Shells
Another point to remember is that atoms are built from the nucleus outwards; this means that electrons must first fill the K-shell (closest to the nucleus) with two electrons before it can start to fill the L-shell, and so on. If you look at any periodic table you will see that every element has an atomic number. The atomic number tells us how many protons are in the nucleus of that element. Hydrogen has an atomic number of one. This tells us that, in its non-ionic form, hydrogen has one proton. We can then conclude that hydrogen also has one electron. As the shells fill up from the inside outwards, this single hydrogen electron must sit on a K-shell. Larger elements like krypton, with an atomic number of 36 (36 protons and 36 electrons), don’t need a mathematical genius to calculate how many shells it has. Filling them up from the nucleus outwards we can calculate that krypton has enough electrons to fill three shells and partially fill a fourth. The K-shell has space for two electrons, the L-shell has space for eight electrons, and the M-shell has space for eighteen (2 + 8 + 18) and can also supply eight electrons to complete a fourth outer shell. The fourth or N-shell will only contain eight electrons and not the maximum 32. That’s a lot of empty space. Which is where another law of science comes in – the octet rule: if an element has eight electrons in its valence shell, it is stable (this does not apply, of course, to the K-shell that only has space for two electrons).
The outermost shell of any atom is called the valence shell and it is in this shell that all bonding occurs. We call the ability of an element to react with another its valence. When a valence shell has less than eight electrons, the atom will try to amend the situation through bonding.
The final point that needs to be understood is how we know if an element is reactive or not. This depends on its valence shell and, thankfully, answering this question is made very simple thanks to the periodic table. Just like we saw before with hydrogen and helium the periodic table places elements into very indicative groups. Helium is found in group 18 of the periodic table of the elements and this group is also known as the inert gases or noble gases group. The word ‘inert’ tells us that helium is not a very reactive element. We also know this because its valence shell contains eight electrons (which we can calculate if we know the atomic number and the maximum number of electrons per shell) and, as the octet rule tells us, a valence shell with eight or more electrons is stable.
Sodium, on the other hand, has an atomic number of eleven. It fills the K-shell, the L-shell, and part of the M-shell (2, 8, 1). There is only one electron in its valence shell, just like hydrogen. This tells us that sodium is highly unstable and will react with the atoms that surround it to get rid of that one electron. Every element in group one of the periodic table is highly reactive. A single atom of hydrogen rarely stays single for long. It either joins another hydrogen molecule to produce H2 or reacts with other elements. In the form of H2, the covalent-bonded valence shells share the ideal maximum – two electrons.
Covalent and Other Bonds
Covalent bond, ionic bond, and metallic bond differences are due to the electrical charges and saturation of the valence shells of different element types and their corresponding groups. The definition of a covalent bond is a chemical bond between two very similarly charged non-metal atoms. In this case, atoms close to one another will share one or more valence electrons (not exchange them) and form a molecule. How many electrons are shared between two atoms determines whether a covalent bond is a single, double, or triple bond. A single covalent bond is formed between two electrons shared between two atoms, a double covalent bond is the result of four electrons being shared by two atoms, and a triple covalent bond – the strongest of covalent bonds – is formed when six electrons are shared between two atoms. A molecule might have a mix of covalent bonds, and an element may bond with more than one atom; however, covalent bonds specifically deal with two atoms sharing electrons. Covalent compounds can be found in gases, liquids, and solids and do not efficiently conduct electricity or heat.
When close to each other, the attraction of each atom’s nucleus for the same electrons forms a bond. In other words, this is less an exchange than a valence electron spending time close to the valence orbit of the other atom. Examples of covalent bonds are dihydrogen (H2), water (H20), methane (CH4), chlorine (Cl2), nitrogen (N2), and carbon dioxide (CO2). Some of these bonds are pictured below.

Ionic bonds (or electrovalent bonds) are different. They involve a bond between two oppositely-charged ions (positive and negative) that is formed when one atom transfers electrons to the other. Remember that covalent bonds share electrons. This is an important difference. In ionic bonding, an atom donates extra electrons to empty its current valence shell (if containing less than eight electrons); the partner atom accepts electrons to help fill its valence shell. It is always important to keep in mind that atoms are consistently attempting to achieve an electron configuration that is as similar as possible to that of the most stable group in the periodic table, the noble gases.
In ionic bonds, one ion is a metal, the other a non-metal. You should also be aware that negative ions bind to positive ions and vice versa. For example, positively-charged sodium ions bond with negatively-charged chloride ions to produce a neutrally-charged salt (Na-Cl) molecule. Lithium (L+) ions form ionic bonds with bromide (Br–) ions to form neutrally-charged lithium bromide (Li-Br).
Metals are found in the earliest groups of the periodic table and this indicates a lack of electrons in the valence shell. It is much easier and more energy-efficient to donate low numbers of electrons and rely on the stability of the shell underneath than to use energy to add seven, six or five extra electrons to a near-empty valence shell. On the other hand, non-metals feature quite late in the periodic table and generally possess six or seven outer shell electrons; this means that non-metal ions are much more likely to receive electrons to form a complete outer shell as this requires the least energy expenditure.
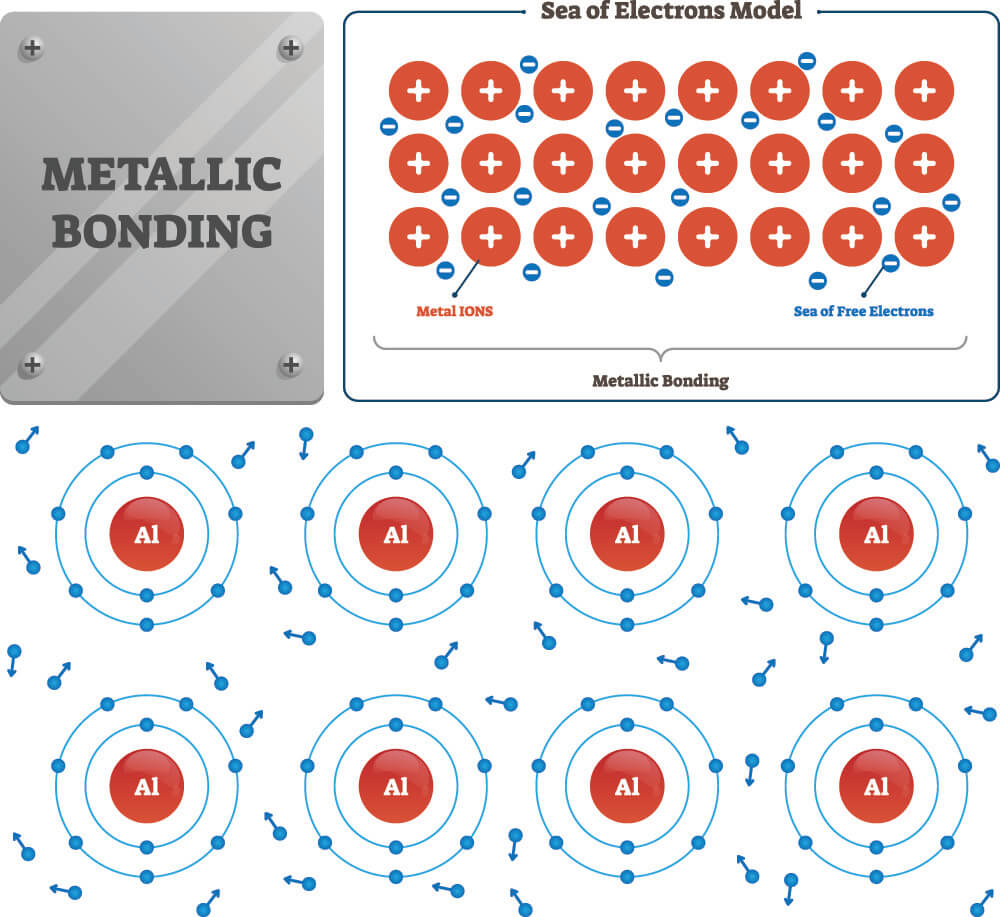
The third type of atomic bond is the metallic bond. This is a series of bonds formed between multiple metal atoms – not metal ions – and is characterized by valence shell electron-sharing between more than one neighboring metal atom. These multiple metal atoms form a lattice-like structure that is surrounded by electrons. These free electrons are not fixed to a single orbit on a single atom but are non-localized. Normally, the positive charges of the multiple metal atom nuclei would repel one another; however, the cloud of surrounding negatively-charged electrons (conduction electrons) keeps these metal nuclei in close formation. It is the conduction electrons that give metals their high thermal and electrical conductivity properties.
For further information about the different types of bonds, have a look at this summary.
How do Periodic Table Groups Help?
Knowing what atoms are metal, metalloid, or non-metal and being aware when an atom is in its ionic form makes understanding and recognizing covalent and other types of atomic bond much easier.
The periodic table is split into groups (or families) and categories. The seven horizontal rows are known as periods. The vertical rows create eighteen columns. These eighteen columns are split into groups I through to VIII (A and B). Looking at the periods of the periodic table does not help much in determining a type of bond. It is the group that gives us the most clues.
Groups tend to have similar characteristics; they form families that behave and look a particular way. However, these categories also give us some additional information. There are eight groups as shown below.
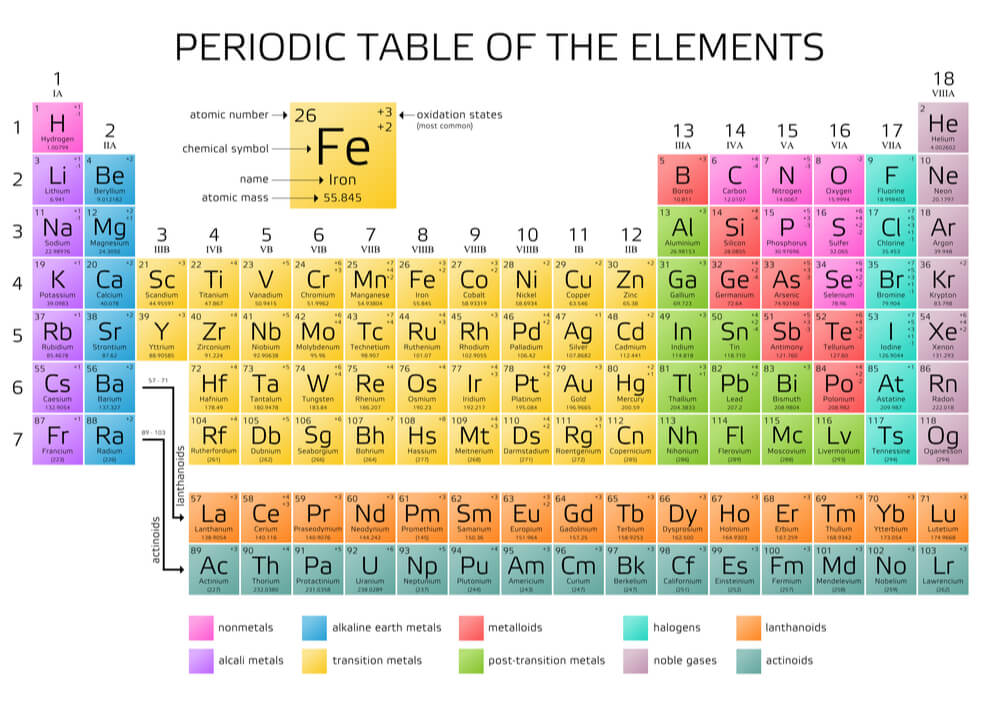
In the image, you will immediately notice that hydrogen seems to be in the wrong place. It is not an alkali metal (it’s a gas) but is listed in group one. This is because the groups tell us how many valence electrons an element has. Group one elements or atoms have one valence electron. Group two members have two valence electrons. If you’re looking at the periodic table and frowning, it’s probably because you are not looking at the Roman numerals IA to VIIIA; these are the group numbers. Group B metals (in yellow) are referred to as transition metals.
The periodic table is also useful when considering nonpolar and polar covalent bonds that describe covalent bonds between similar and not so similar atoms respectively. If two atoms are dissimilar, the chances are high one of them will have fewer electron shells. The valence shell of that atom will be closer to the atom nucleus and slightly stronger than the valence shell of a larger atom. The shared atoms will spend more time in the orbits of the stronger atom. This describes the electronegativity of an element. On the periodic table, the further to the right you travel, the greater the electronegativity. The further down you travel, the lower the negativity.
Covalent Bonds and Hydrogen
Covalent bonds and hydrogen go hand-in-hand. It is always easiest to use hydrogen if you want to keep covalent bonding simple. Hydrogen has atomic number one. We already know that this number indicates a nucleus with one proton and we can surmise that hydrogen also has one electron. With one negative charge (electron) and one positive charge (proton), the hydrogen of the periodic table is a neutral atom, not an ion. It is a gas, not a metal. Hydrogen is, therefore, a candidate for covalent bonding.
As we have already seen, hydrogen does not remain single for long and is always ready to find a partner. This is because its single shell has one atom where there is place for two. H2 is a gas that forms when two hydrogen atoms bind by way of a covalent bond. It is also known as dihydrogen or molecular hydrogen. A single molecule of H2 contains two protons and two electrons. It is the most common form of hydrogen because it is extremely stable. What can be confusing is the term hydrogen bond. This is not a covalent bond and does not describe H2 but specific bonds between a hydrogen atom and fluorine, oxygen, or nitrogen. We won’t look at hydrogen bonds here, just covalent ones.
Dihydrogen molecules form when two H atoms collide. As both atoms’ K-shells only host a single electron, if each atom shares one electron and borrows another, they will both enjoy the stability of a complete valence shell. To form a covalent bond, an element may not be in its ionic form, must be a non-metal or transitional metal, and must be similar in form and charge to its new partner. Furthermore, the non-metal atoms should be partially unstable; noble gases with full valence shells hardly ever form molecules or have ionic forms.
Only when two atoms of the same element form a covalent bond will their electrons be equally shared. If different elements share electrons through covalent bonding, one atom’s electrons will have higher electronegativity (higher pulling power) thanks to the closer distance atom nucleus to its surrounding electrons. The closer a valence shell is to the nucleus, the greater its electronegativity. When one atom forms a bond with an atom of a different type, the result is a polar covalent bond. Where the levels of electronegativity are the same, nonpolar covalent bonds will be formed. You can find separate articles for these types of bonds that describe them in detail. Alternatively, this article gives a short summary that looks at how the presence of electronegativity can determine the covalent bond type.
Quiz
