Chemical Formula Definition
A chemical formula is a notation used by scientists to show the number and type of atoms present in a molecule, using the atomic symbols and numerical subscripts. A chemical formula is a simple representation, in writing, of a three dimensional molecule that exists. A chemical formula describes a substance, down to the exact atoms which make it up. There are three basic types of chemical formula, the empirical formula, the molecular formula, and the structural formula.
Each one of these chemical formula provide slightly different information about the makeup of a substance, and clues to its three dimensional shape and how it will interact with other molecules, atoms and ions. In a chemical formula, the letters represent the atomic symbol of each atom. The subscript (lower) represents the number of each atom, while the superscript (higher) represents the charge on a given atom. A coefficient before a chemical formula represents that many units of the molecule. Each of the different types of chemical formula is read a little differently.
Types of Chemical Formula
Empirical Formula
The empirical chemical formula represents the relative number of atoms of each element in the compound. Some compounds, like water, have the same empirical and molecular formula, because they are small and have the same ratio of atoms in molecules and number of atoms in a molecule. The empirical and molecular formula for water looks like this:
H2O
The empirical formula is determined by the weight of each atom within the molecule. Therefore, for a slightly bigger molecule like hydrogen peroxide, the empirical formula shows only the ratio of atoms. In this case:
HO:
However, this empirical chemical formula only shows the basic foundation of the molecule. In reality, two HO: molecules come together to form a hydrogen peroxide molecule.
Molecular Formula
The molecular formula comes in to show the actual number of atoms within each molecule. Thus, for hydrogen peroxide the molecular formula is thus:
H2O2
As you can see, this somewhat confuses the actual structure of hydrogen peroxide. While the empirical chemical formula gives clues that the molecule has two oxygen atoms bonded together in the middle, the molecular formula does not make that clear at all. However, the molecular formula is often used to describe molecules, simply because it is convenient and most molecules can be looked up after their formula is identified.
Structural Formula
The structural formula of a molecule is a chemical formula with a more artistic twist. In these chemical formula, the actual bonds between molecules are shown. This helps the reader understand how the different atoms are connected, and thus how the molecule functions in space. There are many different structural chemical formula to consider.
The simplest, the electron dot method, uses colons and periods to show bonds between atoms. Each colon represents a pair of electrons, shared between the atoms on either side of the colon. This formula more accurately represents the actual arrangement of atoms within a molecule. In the case of water, the electron dot formula would look like this:
H:O:H
Another chemical formula, the bond-line formula, also shows the bonds between atoms. Instead of showing each electron which is shared, a line is used to designate an electron pair shared between the atoms. Water, in bond-line formula, looks like this:
H-O-H
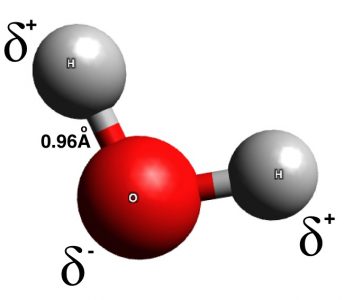
Scientists have come up with much more advanced formula and representations of molecules, including three dimensional ball-and-stick models, space-filling models, and even models which consider the electron density of the atoms being modeled. These advanced models consider not only the atoms present and their number, but the angles, sizes, and distances between atoms within a molecule. The ball and stick model of water, below, even shows the polarity of the molecule, as the large oxygen atom tends to attract the most electrons.
Molecular Mass from Chemical Formula
One important skill derived from the chemical formula is calculating the molecular mass. The molecular mass of a molecule is the sum of all the different atoms within. Each substance has a particular molecular mass, determined by its particular makeup.
To determine the molecular mass of a substance, consider the chemical formula. The formula easily displays each atom present. Be sure to multiply by the number of atoms of each molecule. The subscripts on each atom will indicate how many there are. Some large molecules with multiple similar groups will show the groups something like the example below:
C(OH)4
In this case, there are four groups of (OH), not 4 hydrogen atoms. Make sure you take this into account when calculating molecular mass. The molecular mass can be used to identify substances, properly weigh substances for experiments, and do a number of calculations involving the energy involved in chemical reactions. Scientists often use a chemical formula as a store of much of this information, without having to explain basic chemistry in every paper.
Quiz
References
- Moore, J. T. (2010). Chemistry Essentials for Dummies. Indianapolis: Wiley Publishing, Inc.
- Nelson, D. L., & Cox, M. M. (2008). Principles of Biochemistry. New York: W.H. Freeman and Company.
- Silberberg, M. S. (2009). Chemistry: The Molecular Nature of Matter and Change (5th ed.). Boston: McGraw-Hill Higher Education.