Life depends on water, and cells are very sensitive to the fluid environment surrounding them. They react to the composition of the extracellular fluid (ECF), and with conditions stray outside the range of equilibrium, it can easily cause damage or death. The body strives to keep all its systems in equilibrium and regulating fluid balance begins at the cellular level.
Osmosis
In biology, osmosis is the movement of water across a semipermeable membrane from an area of higher concentration to lower concentration to achieve equilibrium. Osmosis is a spontaneous and passive process that needs no energy, unlike ion pumps in the cell membrane which require ATP (adenosine triphosphate) to function.
The movement of water in osmosis is driven by the difference in solute concentration between the ECF and the intracellular fluid (ICF). Solutes are all the materials dissolved in the ICF and ECF like proteins, ions, carbohydrates, enzymes, fats, etc. It doesn’t matter what is dissolved, just how much is there and whether the amount is higher inside the cell or outside the cell. The difference between the solute concentration of the ICF and the ECF is the osmotic gradient. The osmotic gradient creates osmotic pressure, the force that drives the movement of water.
Isotonic Solution
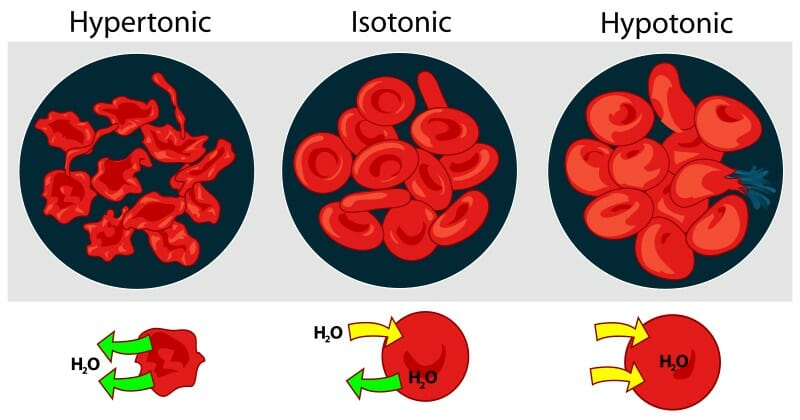
An isotonic solution (for example, the ECF) has the same osmotic pressure as the ICF. Under these conditions, water passes back and forth across the semipermeable membrane to keep the cell in equilibrium with the surroundings. Adding more solute particles to the ECF changes the osmotic gradient so that the osmotic pressure increases inside the cell and more water flows out across the membrane. If not stopped, the cell will wrinkle and eventually shrivel up and die. Conversely, more solute particles in the ICF causes more water to rush into the cell which may cause it to burst.
Plant cells have a cell wall surrounding the plasma membrane. The effect of an isotonic solution is the same but not as obvious because of the rigid wall. There are observable changes in hypertonic and hypotonic solutions, however, if there is a sufficient difference in the osmotic gradient.
Plant cells in a hypotonic solution become bloated as the cell membrane inside swells and presses against the cell wall. A hypertonic solution will make water leave cells, but a plant cell looks more like a pincushion than a wrinkled animal cell. This is because inside, the cell membrane is attached to the cell wall at various points called plasmodesmata. The shrunken cell membrane pulls on the plasmodesmata making the cell wall pinch inward at the points of attachment.
The image above shows what happens to red blood cells in hypertonic, isotonic, and hypotonic solutions.
References
- OpenStax College. (2018). Anatomy & Physiology. Houston, TX. OpenStax CNX. Retrieved from http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@8.119
- Tonicity. (2018, February 8). In Wikipedia. Retrieved from https://en.wikipedia.org/w/index.php?title=Tonicity&oldid=824611413