Base Pair Definition
Base pairs refer to the sets of hydrogen-linked nucleobases that make up nucleic acids DNA and RNA. They were first described by Dr. Francis Crick and Dr. James Watson who are best known for discovering the helical, “twist around,” structure of DNA (1953). At this time, DNA was also identified as the source of transferring material that takes place during cell division. In their model, Watson and Crick predicted that the two strands of DNA are able to intertwine with the help of rule-abiding hydrogen bonds.
Taking a step back, our DNA is composed of four types of nucleobases: adenine, thymine, guanine, and cytosine. Nucleotides can be thought of as the biological “building blocks” that create and sustain life. Each contains a nitrogenous base, a sugar (deoxyribose in DNA), and a phosphate group. The sugar and phosphate groups form the hydrophilic outer “backbone” of the DNA helix, while the nitrogenous bases point toward the nonpolar, hydrophobic core. Adenine and guanine belong to a class called “purines,” while cytosine and thymine belong to the “pyrimidine” group. These bases adhere together following a set of specific base pair rules discussed below.
Pictured is a virtual animation of the double-stranded DNA helix.
Purine and Pyrimidine Groups
- Purine bases = Adenine + Guanine are “PURe As Gold.”
- Pyrimidine bases = Cytosine + Thymine
Base Pair Rules in DNA
Watson & Crick base pairs follow a specific rule of hydrogen bonding. In complimentary pairing, one purine links with one pyrimidine nucleic base. In DNA, specifically, adenine only pairs with thymine to form two hydrogen bonds. In other words, this pair forms a strong “double bond” that ensures the dimers are held together. Cytosine and guanine, on the other hand, form three hydrogen bonds that allow for a shorter and more rigid link. These nitrogenous bases are all planar in nature, meaning that they are fairly flat and rigid molecules. This, of course, has the benefit of making DNA a sturdy structure which is so important as it contains our entire genetic code that we need to protect and preserve.
Another important facet of base pairs is that the resultant dimers are of the exact same dimension and occupy the same amount of three-dimensional space. This allows DNA to assume a “steric fit” that ascertains a uniform helical structure throughout. While the ratios of C+G: A+T nucleotides may vary from organism to organism, what remains true is that the amount of adenine in the organism will always match the amount of thymine, as well as cytosine to guanine (called “Chargaff’s rule”).
Watson-Crick Base Pair Hydrogen Bonds
- Adenine + Thymine = form two hydrogen bonds, between Oxygen/Hydrogen and Nitrogen/Hydrogen.
- Cytosine + Guanine = form three hydrogen bonds, between Oxygen/Hydrogen (2) and Nitrogen/Hydrogen (1).
- Remember: Hydrogen bond donors are only those with H atoms bound to electronegative atoms Nitrogen or Oxygen. Hydrogen bond acceptors are electronegative atoms with at least one pair of lone electrons.
Base Pairs in RNA
While RNA also conforms to Watson-Crick base pair rules, there are some key structural differences to note. There are examples of DNA that is single stranded, and RNA that is double stranded (i.e. RNAi) but typically, RNA is thought of as single-stranded and DNA as double stranded. Other differences to note are that RNA contains ribose as its sugar base, and uses uracil instead of thymine. Since uracil and thymine are structurally similar, they are both able to base pair bond with adenine in a similar way. Likewise, RNA is much shorter than DNA and can be found in many forms including mRNA – which is the incredible molecule that is translated into every protein in our cells and bodies.
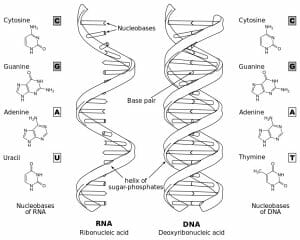
The figure depicts structural differences between RNA and DNA.
Quiz
1. Correctly name the purine bases in DNA:
A. Cytosine, thymine
B. Guanine, adenine
C. Cytosine, adenine
D. Thymine guanine
2. Correctly name the pyrimidine bases in DNA:
A. Cytosine, thymine
B. Guanine, adenine
C. Cytosine, adenine
D. Thymine guanine
3. How many hydrogen bonds do adenine and thymine form?
A. 1
B. 2
C. 3
D. 4
4. How many hydrogen bonds does cytosine and guanine form?
A. 1
B. 2
C. 3
D. 4
References
- Biology Pages (2017). “Base Pairing.” Retrieved 2017-05-09 from http://www.biology-pages.info/B/BasePairing.html
- Center for BioMolecular Modeling (2017). “Hydrogen Bonding in DNA Base Pairs.” Milwaukee School of Engineering. Retrieved 2017-05-10 from http://cbm.msoe.edu/markMyweb/Hydrogen%20Bonding%20Tutorial_MARK.html
- ChemGuide (2017). “Transcription – From DNA to RNA.” The Structure of RNA. Retrieved 2017-05-09 from http://www.chemguide.co.uk/organicprops/aminoacids/dna3.html
- University of Maine UMECHE (2017). “Structure and Properties of Purines and Pyrimidines.” Department of Chemistry. Retrieved 2017-05-10 from http://chemistry.umeche.maine.edu/CHY431/Basics/PurPyrm.html