AP Biology 3.4 - Cellular Energy
This section of the AP Biology curriculum is meant to be an introduction to what energy is, the rules that apply to energy, and how cells must operate in order to maintain these rules, survive, grow, and reproduce. In this section, we’ll look at a broad overview of how cells gather and distribute energy. We’ll also see how the first and second laws of thermodynamics apply to how cells utilize energy. This includes how cells gather energy via photosynthesis in chloroplast and how cells utilize energy via aerobic respiration in the mitochondria. Though later sections of the AP Biology curriculum dive into the complexities of those processes, in this section we will be looking at how cells create highly ordered systems of enzymes and couple exothermic reactions with endothermic reactions to be as efficient as possible to conserve energy.
Video Tutorial
The following video summarizes the most important aspects of this topic!
To watch more tutorial videos like this, please click here to see our full Youtube Channel!
Resources for this Standard
For Students & Teachers
- Overview & Video Tutorial (This article)
- Quick Test Prep
- Crossword Puzzle
For Teachers Only
ENDURING UNDERSTANDING
ENE-1
The highly complex organization of living systems requires constant input of energy and the exchange of macromolecules.
LEARNING OBJECTIVE
ENE-1.H
Describe the role of energy in living organisms.
ESSENTIAL KNOWLEDGE
ENE-1.H.1
All living systems require constant input of energy.
ENE-1.H.2
Life requires a highly ordered system and does not violate the second law of thermodynamics–
- Energy input must exceed energy loss to maintain order and to power cellular processes.
- Cellular processes that release energy may be coupled with cellular processes that require energy.
- Loss of order or energy flow results in death.
ENE-1.H.3
Energy-related pathways in biological systems are sequential to allow for a more controlled and efficient transfer of energy. A product of a reaction in a metabolic pathway is generally the reactant for the subsequent step in the pathway.
EXCLUSION STATEMENT
Students will need to understand the concept of energy, but the equation for Gibbs free energy is beyond the scope of the course and AP Exam.
3.4 Cellular Energy Overview
Go lift something heavy. Then, do it again, and again, and again. Feel your muscles burning? That is literally the feeling of your muscles trying to produce as much energy as fast as possible to maintain homeostasis and allow you to do another repetition.
The energy required in a muscle cell is needed for a very simple reason – energy changes the shape of proteins in the muscle that reach out and grab other proteins. As they retract, the muscle shrinks – allowing you to pick up a heavy object.
While the physical movement of your muscle cell is an action that obviously requires energy because you are doing physical work, other reasons a cell needs energy are less obvious. Cells need energy to build, degrade, store, and release a wide variety of substances. Yet, cells manipulate energy in a manner that is consistent with the laws of thermodynamics – all the way from the sun into the molecules that cells use. You should definitely understand how this works because it will certainly be referenced on the AP test! So, follow along with us and we cover all the basics of cellular energy!
The large majority of energy that flows through the Earth started out as energy bound to photons that originated from the sun. As these photons passed through the chloroplasts of plants, they were stripped of their energy and it was transferred through an electron transport chain and into the bonds of ATP via ATP synthase. This ATP was then used to produce glucose via the Calvin Cycle. This complex flow of energy is the process of photosynthesis, which will be covered in more detail in section 3.5.
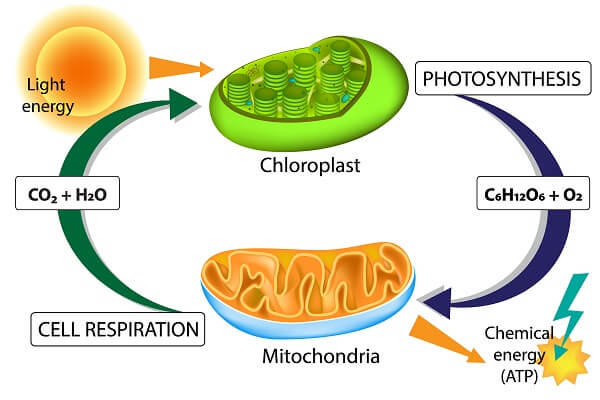
Then, this molecule of glucose will go on to power almost every other reaction in nature. The process of cellular respiration – discussed further in section 3.6 – starts with the breakdown of glucose via glycolysis. The products of glycolysis then enter the mitochondria, where electron carrier molecules are loaded with electrons via the citric acid cycle. These electron carriers then dump their electrons into another electron transport chain, which powers another ATP synthase enzyme. This enzyme produces ATP that is distributed throughout the cell. This convoluted process is cellular respiration.
These ATP molecules created by cellular respiration are utilized by a wide variety of cellular processes. They are used in primary active transport in order to concentrate molecules into various compartments and create ion gradients. These ATP molecules also power the synthesis of macromolecules, cell signaling, and various cell movements. There are literally thousands of processes powered by the combination of photosynthesis and cellular respiration in plants, animals, and fungi. But, if you were paying close attention, you’re probably wondering why plants need cellular respiration at all. In other words, why do plants need cellular respiration if photosynthesis is already creating ATP?
The answer is simple. You can only store so many ATP molecules within the cell before it can no longer be manufactured. The enzymes that create ATP can also complete the reverse reaction, meaning that high concentrations of ATP make it more likely the ATP will be converted back into ADP. Furthermore, cells need energy constantly – even in the dark. Without a storage molecule like glucose, plant cells would quickly run out of energy after the sun went down. Lucky for us, plants make plenty of glucose and store energy in other ways that power the entire food chain!
Though AP Biology does not focus on the physics or specific chemistry of life on Earth, there are a few concepts related to these topics that you will be tested on. For instance, if you know the laws of thermodynamics, it may seem like living organisms are disobeying the laws. But, these wouldn’t be very good laws if all of biology disobeyed them.
Consider the first law of thermodynamics. It states that an isolated system has a finite amount of energy that cannot be created or destroyed, only converted into different forms.
It may seem that a biological organism breaks this law as it continuously grows, incorporates energy into the bonds of newly synthesized molecules, and reproduces. While it is true that organisms constantly acquire energy and store that energy in new molecules, it is not true that an organism represents an isolated system.
In fact, organisms are connected to the energy of the entire universe. The energy within the universe is constantly combining and dissociating into different forms, though none of it is lost.
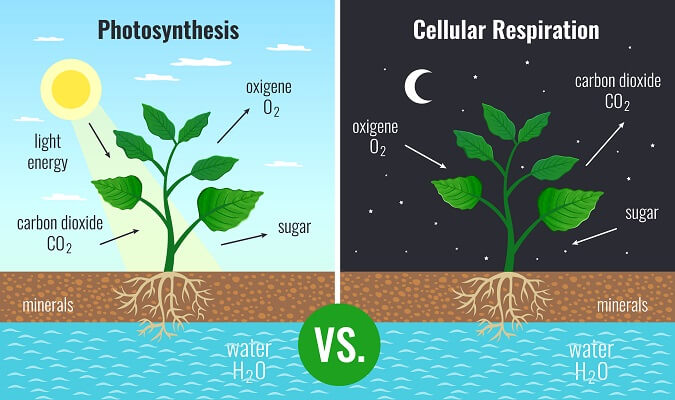
Now, consider the second law of thermodynamics. This law states that systems move from more ordered to less ordered, leading to a maximum amount of entropy. Entropy is a measure of chaos, or a lack of order, within a system. Since organisms are constantly taking smaller molecules and combining them into larger molecules, organisms are technically decreasing the entropy in their immediate environment. But, that is part of the reason why organisms need a constant flow of energy from the sun – organisms are constantly battling the breakdown of larger molecules into smaller molecules and trying to become as ordered as possible. The energy flows from the sun, through the processes of photosynthesis and cell respiration, and back into the universe as it powers processes and is lost as heat. Without this constant input of energy, life on Earth would quickly return to entropy and die off.
With the Laws of Thermodynamics against them, cells have a few tricks up their sleeves to continuously amass more energy, create ordered systems, grow, and reproduce.
First off, one of the most important tools for creating timely, ordered reactions is the use of enzymes. Cells use enzymes to carry out thousands of reactions at different times, in different parts of their cells. The catabolism has hundreds of different enzymes working to break down molecules, while the anabolism has an entirely different set (though there is some overlap).
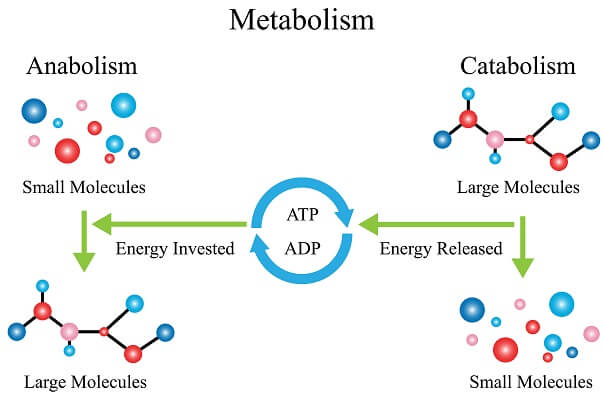
Furthermore, enzymes required for similar processes are often located very close to each other physically, and they are able to quickly access the products from a previous enzyme. This keeps processes very ordered, ensuring that the cell ends up with the right product at the end of a complex process that can include dozens of individual enzymes.
Enzymes are also important because they lower the amount of activation energy that a particular reaction needs to get started, but they don’t affect the energy released or absorbed by individual reactions. Another trick cells use is coupling exothermic reactions with endothermic reactions in order to utilize the energy released by the first to power the latter. So, by ordering enzymes and specific reactions, cells can be as efficient as possible as they battle the laws of thermodynamics.
To better understand how cells maintain order and energy flow, let’s go back to the first example we used to start this lesson: muscle cells.
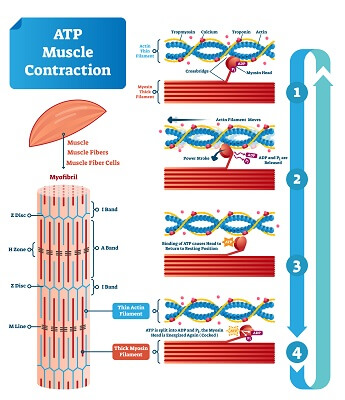
Every time you flex your muscle, bands of proteins in each muscle fiber shorten, causing entire muscles to shorten. In order to do this, the protein strands require ATP energy in order to move. An ATP molecule binds to the protein myosin, which catalyzes the release of energy as ATP is converted to ADP. The energy causes the myosin head to lurch forward. The myosin head attaches to the thin actin filament, and as the myosin protein returns to its normal position it completes a “power stroke.” This pulls the strands of actin and myosin together, shortening the muscle. This process continues as long as you continue to flex your muscle, which clearly uses large amounts of ATP.
Though your cells store a small amount of ATP, any prolonged flexing means your muscles will quickly run out of energy. So, the mitochondria in muscle cells are quickly working to replace the ATP that was used up. Using a highly ordered series of enzymes, the Krebs cycle is quickly pumping out electron carriers and small amounts of ATP. These electron carriers quickly make their way to the inner mitochondrial membrane and the electron transport chain, where they use the energy they are carrying to create a hydrogen ion gradient. This gradient powers ATP synthase, which catalyzes the formation of ATP molecules that can be distributed to a cell. Essentially, this highly ordered process is collecting the energy from many exothermic reactions to create ATP – a highly endothermic process.

While this system is enormously complex, there is one fatal flaw that can shut down the whole thing. A lack of oxygen. Oxygen is the final electron acceptor for the electron transport chain. Without oxygen, the whole conveyor belt of reactions stops, beginning with the electron transport chain and working its way back to the many reactions of the Krebs cycle.
Luckily, cells have a backup generator. When there is no oxygen, cells revert to a completely different set of enzymes to complete the completely separate process of lactic acid fermentation from remaining glucose molecules. This process produces much less ATP, but it allows the cell to maintain order and continue functioning until the body can supply oxygen again.
Phew! That was a lot! But, hopefully, it shows how energy flows within a typical cell and how cells combat the laws of thermodynamics by maintaining a highly ordered system of enzymes that couple energy-releasing reactions to reactions that need energy input! Don’t worry about the details or specifics, we will cover those in later sections of the AP Biology curriculum.
