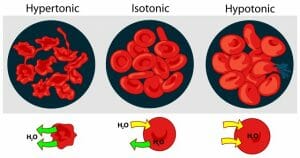
Tonicity Definition
Tonicity is the concentration of a solution as compared to another solution. Concentration describes the amount of solutes dissolved by a solution. If a solution has a higher concentration of solutes (less water) than another it is said to be hypertonic. A hypotonic solution has a lower concentration of solutes and more water than another solution. Isotonic solutions contain the same concentration of solutes. In biology, the tonicity of the environment compared to the cell determines how water moves across the semipermeable membrane. The graphic below shows the tonicity of different environment, and which way water moves. Remember, water moves to balance the concentration gradient of the solutes. It will move from high solute concentration to low solute concentration.
The ability of water to dissolve solutes is due to its polar nature. The differing electrical poles on each water molecules cause temporary hydrogen bonds to form between other water molecules and solutes. This distributes solutes and moves water into new regions. The tonicity of the water is increased as solutes are introduced, but to label the solution with one of the specific terms of tonicity can only be done when comparing the solution to another. Likewise, two different solutes take different amount of water to dissolve. One solute, like silicon, may take many water molecules to dissolve while another may be completely dissolved even in high concentrations. The overall tonicity of water can be described by the osmolarity, which is the total concentration of all dissolved solutes.
Related Biology Terms
- Hypotonic – When one solution contains more water and less solutes than another solution.
- Hypertonic – A solution of higher concentration that the solution it is being compared to.
- Isotonic – Two solutions that exist with the same solute concentration.
- Osmolarity – The concentration of a solution in parts of solute divided by volume of water.
Quiz
1. A plant cell is placed in an aqueous solution. Water floods the cell, and the cell becomes rigid with pressure. What is the tonicity of the solution compared to the cell?
A. Isotonic
B. Hypertonic
C. Hypotonic
2. Two identical solutions contained in narrow beakers are separated by a semi-permeable membrane. If salt is added to one beaker, increasing its tonicity, what will happen to the volume of water in the beaker?
A. It will increase
B. It will decrease
C. It will stay the same