Proteolysis Definition
Proteolysis is the hydrolysis of the peptide bonds that hold proteins together, resulting in the breakdown of proteins into their key components, peptides and amino acids. Proteolysis can occur as a method of regulation of cellular processes by reducing the concentration of a protein, transforming a protein into an active form, or by providing amino acids required to synthesize a different protein. Proteolysis is often performed by proteases, enzymes that catalyse the breakdown of proteins. It can also occur as a result of adverse cellular conditions such as extreme temperature, acidity, or salinity, which disrupts the molecules in the peptide bonds and results in the bonds breaking.
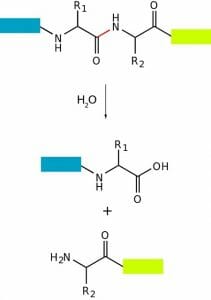
This figure depicts the breakdown of a peptide bond into the constituent amino acids through hydrolysis of the amide bond.
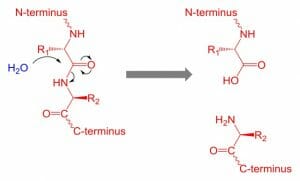
This figure depicts the hydrolysis of a peptide bond through the addition of water.
Proteolysis Structure
Proteolysis occurs when the peptide bonds holding a protein together are hydrolyzed. This often occurs through catalysis by proteases, enzymes that are involved in the breakdown of proteins. The enzymes interact with proteins with substrate specificity based on the conformation of the proteins and the amino acid residue to which they attach. Proteases function in one of two ways: they can break the amide bond between two amino acids at the amino or carboxy terminal (exopeptidases), or they can cleave the protein within the substrate (endopeptidases). Proteases can be categorized into six types, based on the way in which they hydrolyze proteins and the residue which is involved: aspartate, cysteine, glutamate, metallo, serine, and threonine proteases. The different categories hydrolyze the amide bond either through an addition/elimination reaction that produces an intermediary, or by direct hydrolysis of the bond by a polarized water molecule. Proteases, and therefore proteolysis, can be inhibited by binding of inhibitors to the protease active sites, or through spatial confinement separating them from their substrates. This inhibition plays an important role in regulation of cellular processes.
Proteolysis Function
Proteolysis plays a number of important yet diverse roles in the body. It can control protein function either by producing or removing proteins. Proteolysis can regulate the concentration of a protein by removing any excess protein such that its function is diminished or impaired. Proteolysis is often responsible for complete inactivation of proteins, which can result in disruption of protein-protein interactions and signalling cascades including apoptosis. Alternatively, proteases can produce active proteins by conducting fine-scale modifications to a non-functioning or proto-protein, or by altering its physical state or location. Examples of this include regulation of blood clotting through the removal of clots by plasmin and fibrinolysis, and the activation of the protease trypsin by modification of the pre-protease zymogen.
Proteolysis also acts as cellular clean-up process by removing damaged or unnecessary proteins. Damaged proteins include those that have been fractured by another mechanism leaving only a protein fragment and misfolded proteins that are functionally impaired. The proteases will recycle the amino acids to make new or more appropriate proteins, or to relocate the proteins to a new region. Proteins that are intact and folded correctly will not be broken down, essentially acting as a cellular quality control function.
Proteolysis is a key component of nutrient digestion, breaking down any protein ingested so that the nutrients are available to be taken up by the organism. In this process the proteins are completely broken down into their amino acids. Proteolysis is also a component of food production, with proteases breaking down milk proteins in cheese production, and a low pH driving proteolysis of actin in dry sausage fermentation.
Quiz
1. How does proteolysis break down a protein?
A. hydrolysis
B. dehydration
C. combustion
D. acid-base
2. What is an important function of proteolysis?
A. organogenesis
B. formation of blood cells
C. removal of toxins
D. regulation of proteins
3. What causes proteolysis?
A. enzymes
B. high temperatures
C. extreme acidity
D. all of the above
References
- Kaiser, M., Huber, R., & Ehrmann, M. (2013). “Proteolysis”. Brenner’s Encyclopedia of Genetics, 2nd ed. 501-503.
